Mineralogy Lecture 16
advertisement

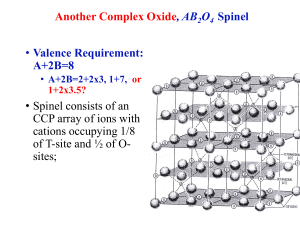
Introduction to Mineralogy Dr. Tark Hamilton Chapter 4: Lecture 16 The Chemical Basis of Minerals (Perovskite & Spinels) Camosun College GEOS 250 Lectures: 9:30-10:20 M T Th F300 Lab: 9:30-12:20 W F300 Perovskite CaTiO3 Structure CCP Orthorhombic 2/m2/m2/m, dipyramidal Structure of Mesosphere! Large A site cation Replaces ¼ of Oxygen Ti+4 octahedral Sharing apices Arborescent Perovskite CaTiO3 Stoltz Quarry, Graulai, Germany Graulai, Germany, Sephan Wolfsried Dysanalite (Nb,REE) Ettringer-Bellerberg Mt.,Germany ~1mm - Stephan Wolfsried Oka, PQ w/ Calcite & Monticellite P.Cristofono Lohley, Germany, Sephan Wolfsried Spinel AB2O4 Structure (~CCP) Alternate layers parallel (111) Octahedral & Octahedral - Tetrahedral Oct Oct & Tet Oct CCP with 1/8 Tetrahedral = A ¼ Octahedral = B Perpendicular to (111) after Waychunas (1991) Normal Spinel: B all Oct Inverse Spinel: B ½ Tet Spinel Structure (001) after Steven Dutch Layer 1 View along Four-fold Symmetry Axis (001 Plane) Layer 3 Filled octahedra form criss-cross rows with alternating layers of parallel rows offset as shown on the right side of the diagram. The square holes enclosed by the rows of octahedra are filled with tetrahedra Spinels: 2 Types of sub-unit cells Figure 1-a: Two kinds of occupied tetrahedral sites in spinel sub-cell a. A is in green and O is in red. Figure 1-b: Occupied octahedral site in spinel subcell b. B is in gray, and O is in red. Figure 2: Arrangement of structure a and b in one unit cell. shaded one represents structure a, while white one represents b. Gary Wulfsberg, Inorganic Chemistry, (2000) Spinel Formulae: A+2B+32O4 > (Y+4X+22O4 Olivine ~12% less dense: transition 360-610km) • Normal Spinels: • Inverse Spinels: • • • • • • • • • • • • Spinel: MgAl2O4 Hercynite: FeAl2O4 Gahnite: ZnAl2O4 Franklinite: ZnFe+32O4 Chromite: FeCr2O4 Magnesiochromite MgCr2O4 LiMn2O4 Lithium battery Magnetite: Fe+2Fe+32O4 Ulvospinel: Fe+22Ti+4O4 Ni+2Fe+32O4 Co+2Fe+32O4 Ferrofluids paramagnets • Thiospinels: • Greigite: Fe+2Fe+32S4 • Cuprous Ferrites CuCr2S4 0.8 < A < 1.1 Ang. (Mg, Fe, Mn, Zn, & Cu) & 0.75 < B < 0.9 Ang. (Ti, Fe, Al, & Co) Magnetospirillum magnetotacticum makes Greigite magnetosomes for navigation Named for ”Magnesia”, Greece C. Thompson Spinels: Mogok, Myanmar 8mm Magnetite with Epidote Rob Lavinsky Franklinite, Sterling Hill with Zincite & Calcite M.Baum 1993 Speen Ghar, Afghanistan Rob Lavinsky Chromite bands in serpentinized Dunite Sommergraben, Austria, Franz Bernhard Named for Ben Franklin & Franklin Furnace Greigite (Fe2+Fe3+2S4) infilling wood Calcite NGHP: Silt Krishna-Godarvi Basin Exsolution of Cubic “Fe-Ti Spinel” & Hexagonal Imeno-Hematite (Norway) Oxygen Linkages in Common Silicates Nesosilicates: Olivine Garnet, Zircon Kyanite (SiO4)-4 Sorosilicates: “Pyro” Lawsonite, Epidote Melilite, Hemimorphite Vesuvianite (Si2O7)-6 Cyclosilicates: Beryl Cordierite, Benitoite Tourmaline (Si6O18)-12 I-Inosilicates: Enstatite Acmite, Augite, Jadeite Wollastonite (Si2O6)-4 II-Inosilicates: Hornblende Arfvedsonite (Si4O11)-6 Phyllosilicates: Paragonite Kaolinite Polylithionite (Si2O5)-2 Tectosilicates: Quartz, Tridymite Coesite (SiO2)0