View - Pondicherry University
advertisement

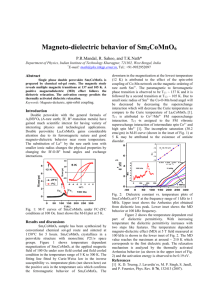
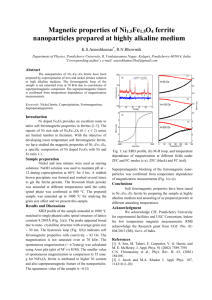
Annealing effects on the structure and ferromagnetic properties of as prepared Co2.25Fe0.75O4 ferrite Manas Ranjan Panda*, and R. N. Bhowmik Department of Physics, Pondicherry University, R. Venkataraman Nagar, Kalapet, Pondicherry-605014, India *Corresponding author’s e-mail: manasranjan056@gmail.com,Mobile: +91-7845794468 Abstract Experimental The material was prepared by co-precipitation of Co(No3)2.6H2O and Fe(NO3)3.9H2O solution in high alkaline medium (pH 11) at 80 0C for 4 hrs. The as prepared sample after annealing at 200 0C, 500 0C, 800 0 C, 900 0C, and 1000 0C in air for 4 hrs was denoted as CoFe2A4, CoFe5A4, CoFe8A4, CoFe9A4, CoFe10A4. Results X-ray diffraction (XRD) pattern showed single phased cubic spinel structure (a 8.200 Å) for TAN 900 0 C and splitting of the cubic spinel structure into Co rich (a 8.095 Å - 8.122 Å) and Fe rich (a 8.302 Å - 8.327 Å) phases for other annealing temperatures. The sample annealed at 1000 0C showed a minor trace of CoO, as seen in earlier work [3]. Major XRD peaks were fitted with Gaussian shape to obtain area, height, half width and positions of the peaks. Grain size of the material varied the range 5-25 nm by increasing TAN 200 0C to 1000 0C. Percentage of the Co- rich and Fe-rich phases was calculated from the peak height and area ratio after resolving two phases using profile fitting, and shown at different TAN in the inset (a) of Fig. 1. Raman spectra of the samples suggested normal spinel structure (more divalent cations in A sites and trivalent cations in B sites). Field dependent magnetization [M(H)] curves showed room temperature ferromagnetism in all samples. The sample annealed at 800 0C (least Fe-rich phase in inset (b)) showed maximum coercivity 568 Oe and also squareness 0.33.The single phased sample shows lowest coercivity and ferromagnetic moment. 10 Phase (%) 15 CoFe10A4 80 CoFe2A4 Fe-rich phase CoFe8A4 60 CoFe5A4 40 CoFe9A4 Co-rich phase 5 20 200 400 600 0 TAN ( C) 800 1000 0 -5 -10 -15 Inset (b) 600 400 15 M16 kOe HC 10 HC (Oe) 20 Single phase Spinel ferrites are interesting for applications in magnetic recording and electro-magnetic devices [1]. The basic need is ferromagnetism with reasonably large spontaneous magnetization, coercivity and squareness. These parameters are highly tunable in Co rich side (x ≥ 1) of CoxFe3-xO4 [2], where ferromagnetic parameters strongly depend on Co content, distribution of Co and Fe ions among tetrahedral (A) and octahedral (B) coordinated sites, and super-exchange interactions [3]. Material synthesis also played a crucial role in tailoring ferromagnetic parameters in CoxFe3-xO4 ferrite [4, 5]. Inset (a) 100 M at 16 kOe (emu/g) Introduction 20 Magnetization (emu/g) Co2.25Fe0.75O4 ferrite was prepared by co-precipitation route. Depending on annealing temperature (TAN), the as prepared material was stabilized in single phased and bi-phased cubic spinel structure. The samples showed ferromagnetic properties at room temperature and ferromagnetic parameters depends on phase formation. 200 5 20 -20 -15000 -10000 30 40 50 60 Fe rich phase (%) -5000 0 5000 Magnetic Field (Oe) 10000 0 15000 Fig. 1. M(H) curves for different samples. Inset shows the phase (%) with TAN (a) and variation of magnetic parameters with Fe rich phase (b). Conclusions The probable cation distribution of the single phased compound is (Coα2+Fe1-α3+)A[Co2+1-αFe3+2-x+αCo3+x-1]BO4 with x = 2.25 and α 1 (normal spinel structure). In biphased samples, cation distribution in Co-rich and Ferich phases are suggested as (Co2+)A[Fe3+0.75-Co3+1.25+]B O4 and (Co2+)A[Fe3+0.75+Co3+1.25-]BO4, respectively. This work suggest that ferromagnetic properties can be tailored by controlling the single phase and bi-phased structure of the Co rich side of CoxFe3-xO4 compound. Acknowledgment We thank to CIF, Pondicherry University and RRCAT Indore for material characterization. We acknowledge the Research grant from BRNS (No. 2011/37P/45/ BRNS/2628) and UGC (No. 42-804/2013 (SR), Gov. of India. References [1] V.G. Harris, A. Geiler, Y. Chen, S. D. Yoon, J. Magn. Magn. Mater. 321 (2009) 2035-2047. [2] R.N. Bhowmik, V. Vasanthi, and A. Poddar, J. Alloys Compd. 578 (2013) 585-594. [3] H. L. Trong et al., J. Magn. Magn. Mater. 334 (2013) 66-73. [4] R. N. Bhowmik, A.T.Satya, A.Bharathi, J. Alloys Compd. 559 (2013) 134-141. [5] R.N. Bhowmik, Mater. Res. Bull. 50 (2014) 476-482.