ask
advertisement

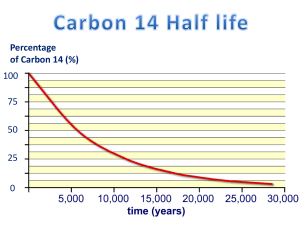
ask-a-scientist created by Dr. John Campbell The 5000 year-old-man, the `ice mummy’ was dated by carbon-14 dating. What is this and how does it work? Edward Winter, Heaton Normal Intermediate School. Scientist Tom Higham, a radiochemist then with the Radiocarbon Dating Laboratory at Waikato University, responded: A living organism is constantly incorporating carbon into its body through food uptake, which builds bones, skin and hair. A small part of the carbon we consume is called `radioactive’ carbon or simply radiocarbon. Radioactive means that it has an unstable atomic structure. This means that after a certain period of time, carbon-14 decays or disappears. As long as a living organism is taking up carbon, it is keeping the carbon-14 in its body at a constant level, but when death occurs, the carbon14 begins to disappear and is not replaced. In the 1950s, scientists discovered that carbon-14 disappears at a known rate. They found that every 5568 years, half the carbon-14 left in the remains of an organism has gone (the radiocarbon `half-life’), so by measuring the carbon14 remaining, they were able to calculate independent ages for carbon samples from archaeological sites. We can date carbon samples from today, back to about 60 000 years ago using this method. Tiny fragments of the Iceman’s bone, skin and grass from his boots have been dated in two radiocarbon laboratories, in Oxford and Zurich. (A piece of grass or skin about the size of one grain of rice is needed for a date). The dates placed the age of the Iceman between 3400-3100 B.C., or about 5500 years ago, the oldest example of a well-preserved human body ever found. For further information: questions@ask-a-scientist.net