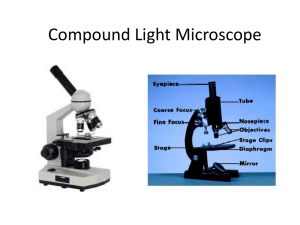
6d) Provide examples DIC is often compared with phase contrast
advertisement

Samenvatting Beeldvormende Technieken 2014 3D-vision 1a) Which physiological mechanisms play a role in 3D-vision? Explain how this works. There are four physiological mechanisms. Accommodation The lens of the eye changes in shape and thickness when looking at objects at different distances. Tiny muscles in the eye make this possible and with this actually change the shape of the cornea. This also changes the refraction of light travelling from air to eye, so that it forms a sharp image on the retina. For accommodation only one eye is needed, but it works together with convergence in order to perceive depth. Convergence Convergence is the angle made by the two viewing axes of a pair of eyes. It is associated with the eye muscles. The muscle tone can be used to estimate the distance to an object. The convergence of the axes of the eyes depends on the distance to the object. The angle between the axes of the eyes increases when an object is very close and decreases when an object is farther away. Binocular differentiation Binocular differentiation means that an object has different image locations on the retina due to the distance of that object from the eyes. When the eyes focus on an object, the optical axes form a parallactic angle. This angle depends on the distance of this object from the eyes. The angle will decrease with increasing distance. So, depth perception becomes more difficult with increasing distance. When you look at two of the same objects at different distances, you can estimate relative distances by comparing the different locations on the retina. This mechanism is based on the natural horizontal separation of the eyes. This is why you need 2 eyes to use this mechanism. Motion parallax Motion parallax: Using the movement of objects to estimate how far objects are away in the scene you are looking at. Objects that are closer to the eye appear to move faster through our field of sight than objects farther away, even if the objects are moving at the same speed. Moving the head of the one observing can cause the same effect. This type of mechanism is important for organisms that only have the use of one eye. 1a) What is chromostereopsys? How does it work? Figure 1. The mechanism in which the moving objects or the movement of the viewers head are used in estimating the distance of objects in the field of sight. Chromostereopsys Chromostereoscopy, is a method to see depth based on the visual phenomenon of chromostereopsis. This system uses colors to guess distance. The eye is not uniformly covered in rods and cones. The cones are concentrated in an area called the fovea. As a result the visual axis isn’t the same as the central axis of the eye. Light enters the eye and lands on the fovea at an angle. Light of shorter wavelengths (blue) is refracted more than light of longer wavelengths (red)(figure 2). That is way the colors appear to be at different positions (figure 3). Figure 1. Light of shorter wavelengths(blue) is refracted more than light of longer wavelengths (red). Figure 2. Blue objects appear to be further away than red objects. 1b) Which psychological mechanisms play a role in 3D-vision? Explain how this works. The six major psychological mechanisms that play a role are: Retinal image size Linear perspective Aerial perspective Overlapping Shade and shadows Texture gradient These mechanisms are all based on experience and learning. In normal imaging these cues are used together to get an image of the surroundings. Retinal image size: the amount of space an object takes on the retina. If the retinal image size of an object is bigger, it looks closer than an object with a smaller retinal image size. The accuracy by which we can estimate the distance from the object depends on our knowledge of the real size of the object. Linear perspective: the gradual reduction of image size when the object goes further away from you. This mechanism can be illustrated with the Ames room. If you look through a hole into the room, it looks normal. But in reality the left corner is further away than the right corner. When a person walks from the left to the right corner he looks bigger by the time he comes closer to the right corner. An example of linear perspective in normal situations is a road that disappears at the horizon. The road seems to get less wide when it approaches the horizon. Aerial perspective: the background of an image becomes fuzzy and blue. The bluer it gets, the further away it looks. This is because the refraction of the light. So distant objects look fuzzier. Atmospheric tinting means that when the whole image is fuzzier, everything looks further away. Overlapping: constant lines look closer than interfered lines. When a person overlaps another person, you know the first person is closer than the second person. Shade and shadows: there is not much difference between shade and shadow. Most of the light comes from above and the image you get is based on this knowledge. For instance, when a round circle is black on the upside and light under this, it appears as hollow. When you turn the image upside down the circle looks like it comes to you. This is dependent on your interpretation on how the light is supposed to fall. Texture gradient: the closer an object gets, the more detail you’ll see. Further away, the texture becomes smoother. This is called a texture gradient. PET en SPECT 2a) What is tomography? Tomografie is het samenstellen van een 3D-model door het stapelen van 2D-plakken. Deze 2D-beelden kunnen bijvoorbeeld worden verkregen door het gebruik van energie/radiatie golven. Er zijn verschillende soorten tomografie, die grofweg kunnen worden verdeeld in twee categorieën: structurele en functionele beeldvorming. Structureel: CT, MRI Functioneel: PET 2a) Explain the physical background of positron emission. What are they? Bij een PET-scan gaat het om de radioactieve verval van de atomen. Een onstabiele kern ondergaat radioactief verval om te kunnen stabiliseren, hierdoor krijgt hij de perfecte proton-neutron verhouding. Een vorm van verval is met positronen. De positron is eigenlijk de tweelingbroer van de elektron, het heeft dezelfde massa als de elektronen. Het enige verschil is dat de positron positief geladen is. Bij de PET scan wordt gebruik gemaakt van het verval. Er wordt een stof ingespoten die gelabeld is met een positron emitting radionuclide, het heeft een instabiele kern. De radionuclide zal vervallen en hierbij komt een positron vrij. De positron botst met een elektron en ondergaat een reactie, hierbij komen 2 fotonen vrij, deze worden gedetecteerd. 2a) Which isotopes emit positrons? Why? Er zijn een aantal grote isotopen die van nature positronen uitschieten. Deze isotopen hebben van henzelf een te grote positieve lading en schieten daarom een positron uit om stabiel te worden. Hieronder staan de 4 meest gebruikte isotopen en hun halveringstijd. • • • • Carbon-11 Nitrogen-13 Oxygen-15 Fluorine-18 half-life: 20.334 min half-life: 9.97 min half-life: 122.24 min half-life: 109.771 min De halveringstijd is de tijd waarin de helft van de isotoop uiteen is gevallen. De isotopen worden gebonden aan een tracer die metabool actief is. Dat wil zeggen dat de tracer wordt opgenomen door de cel en wordt gebruikt in het metabolisme van de cel. De tracer wordt samen met het isotoop intraveneus ingebracht. Voorbeelden van een tracer zijn glucose en methionine. De tracer glucose wordt bijvoorbeeld gebruikt bij het opsporen van kankercellen omdat deze meer cellen meer glucose gebruiken in hun metabolisme dan normale cellen. 2a) What is a voxel and a pixel, what is a look-up table? Demonstrate the effects of various look-up tables. Een pixel is een twee dimensionaal vierkantje wat informatie geeft over een bepaald gegeven. Wanneer er meerdere pixels op elkaar gestapeld worden vormt zich een drie dimensionaal vierkantje wat een voxel genoemd wordt. Om de gegevens in een voxel te kunnen verwerken is een look-up table(LUT) nodig. Meestal wordt er een lineaire look-up table gebruikt. Maar om de kwaliteit van het beeld te verbeteren kan de look-up table worden aangepast. Dit is te zien in de volgende afbeeldingen: Width = 1153, level = 576 Width = 280, level = 860 Width = 320, level = 730 Width = 1, level = 470 2b) What is annihilation? The technique of PET scanning is based on photons that are detected by registering the resulting gamma rays. To obtain these photons, a certain process is needed, namely annihilation. It all starts with a proton-rich isotope (PET radio-isotopes) with an instable nucleus. This nucleus decays via positron emission, which causes a proton in the nucleus to become a neutron. Positron and neutrinos are formed (neutrinos don’t interact with matter, so they can be neglected in PET). You can imagine a positron as a positive charged electron. The positron travels through the body where it interacts with electrons. This process costs energy and when the energy is low enough, the positron and an electron collide with each other. The two particles annihilate each other. Annihilation is the conversion of the mass of an electron + a positron into light, according to E=mc^2. So the process of annihilation produces two photons and these protons travel in opposite directions (anti-parallel). The detection of the photons is possible because of the presence of electromagnetic radiation. (Not visible by eye, the wavelength is to short) Thus, by annihilation photons are released and can be measured by a detector because of the presence of electromagnetic radiation. 2b) What is the mean free path? The mean (not main) free path is the distance between the location at which a positron emerges and the location where it has dissipated enough of its energy to be able to react with an electron to yield annihilation. Easier to imagine as the distance a positron travels before annihilation, it’s about 1 mm. 2b) How is a PET camera composed? A PET camera is composed of detectors that are coupled to photomultiplier tubes. These photomultiplier tubes are connected to a computer system, which creates multicoloured two- or three-dimensional images. When the detector registers an incident gamma photon produced by annihilation it generates a timed pulse. These pulses are combined in coincidence circuitry. The pulses are considered to be coincident if they fall within a short time-window. So, signals that are detected at the same time are registered as coincidence events; the signals come from the same annihilation. 2b) What is the spatial resolution of PET? The main free path of positron emission results in a worse spatial resolution, because the site of emission differs from the site of annihilation. The positrons travel a while before annihilation. Thereby the detector measures not the exact position of the nucleus (site of emission), but it measures the position of annihilation. Also the size of the detector influences the spatial resolution. 2b) What is the temporal resolution? The temporal resolution refers to the accuracy of the measurement time wise. The temporal resolution of PET is very low in reference to other types of measurements. This is due to the fact that PET makes use of nucleus delay, which takes several minutes to set in. This causes the resolution to be tens of seconds to minutes, which severely affects the amount of measurements one can do. 2b) Why is PET still of interest? PET scans, unlike CT or MRI scans, can measure cellular-level metabolic changes occurring in an organ or tissue. This results in an advantage for PET over other technologies to detect whether or not a lesion in the body is benign or malignant. This can sometimes eliminate the need for surgical biopsies if PET scans rule out malignance. This advantage also helps detecting diseases by recognizing disease processes at the beginning with only functional changes, whereas CT or MRI won’t detect any changes until they cause structural changes in the organ or tissue. 2b) What is SPECT? SPECT is an abbreviation of Single-Photon Emission Computed Tomography. SPECT is an imaging technique that shows how blood flows to tissues and organs. While SPECT shows a lot of similarities to PET, SPECT is different in the use of its radioactive isotope. SPECT uses a radioactive isotope, which directly emits a gamma ray upon radioactive decay. This results in one ray instead of two, as with PET, which causes a lower spatial resolution in SPECT images as opposed to PET. However, partly due to more obtainable and more sustainable isotopes used in SPECT, this technique is much less expensive than PET. MRI and fMRI 3a) Explain how protons behave in a magnetic field. Protons are positively charged hydrogen nuclei with a small mass and a North and South Pole. A proton spins around its own axis randomly and therefore creates a magnetic field. In the absence of an external magnetic field, the proton will not point in a specific direction but will just tumble randomly (figure 1). When placed in a magnetic field of an external source, the proton will align or anti-align with the direction of the field in a ratio of approximately 1:1 (figure 2). There should be noted that the protons never align exactly with or against the magnetic field; there is always a small divergence between the axis of the proton and the axis of the field. The protons constantly switch orientation and when there are enough protons present, there will be a slight excess of protons in the aligned orientation creating a magnetic vector oriented along the axis of the external field. This is because the energy-state in which the aligned protons are, is lower and therefore energetic more favourable than the energy-state in which the anti-aligned protons are; these differences get larger when the external magnetic force increases (figure 3). 3a) Subsequently the protons are exposed to electromagnetic radiation (a radio wave). What can happen to the proton? Under what conditions does this occur? When an electromagnetic radio wave (RF) with a frequency equal to the precession frequency (the Larmor frequency) is applied, protons will absorb a specific amount of energy. As a consequence one single proton will jump to a higher energy state. Therefore the magnetization vector (Mø), which is the total magnetic field of the excess protons, will spiral down to the XY plane (Figure 4). Only when the frequency of the radio wave is exactly equal to the precession frequency, the magnetization vector will spiral down. Figure 4 3a) What is the Larmor frequency? When placed in a magnetic field, the protons align either parallel or antiparallel (view above). The protons spin around the straight line of the magnetic field which causes a difference between the axis of the proton and the axis of the magnetic field. This is called precession (figure 5). The Larmor frequency is the frequency of the precession of a charged particle when its motion comes under the influence of an applied magnetic field and a central force. The Larmor frequentie can be calculated by the so called Larmor equation: Where ω is the Larmor frequency in MHz, γ is the gyromagnetic ratio in MHz/Tesla and B is the strength of the static magnetic field in Tesla. The gyromagnetic ratio is the ratio between the magnetic moment and the angular momentum (the amount of rotation an object has). 3a) What happens if the radio wave is turned off? When the radiofrequency source is switched off, the magnetic vector returns to its resting state and this causes a signal (also a radio wave) to be emitted. This signal is used to create the MR images. The hydrogen protons give back their absorbed energy and return slowly to their natural alignment. The emitted energy can be measured. With this data it is possible to determine the density of the protons. When they do this, they give off a signal that the coils pick up an send to the computer system (step 4 and 5; figure 6). Receiver coils are used around the body part in question to act as aerials to improve the detection of the emitted signal. The intensity of the received signal is then plotted on a grey scale and cross sectional images are built up. Once the RF transmitter is turned off three things begin to happen simultaneously. 1. The absorbed RF energy is retransmitted; 2. The excited spins begin to return to the original Mz orientation; 3. Initially in phase, the excited protons begin to dephase. 1. Once Mz (a magnetization vector) has been tipped away from the Z axis, the vector will continue to process around the external Bø field at the resonance frequency ωø. A rotating magnetic field produces electromagnetic radiation. Since ωø is in the radio frequency portion of the electromagnetic spectrum the rotating vector is said to give off RF waves. The absorbed RF energy is now being retransmitted, thereby producing the NMR signal. 2. The process of giving off RF energy occurs as the spins go from a high-energy state to a low-energy state, realigning with Bø. The RF emission is the net result of the Z component (Mz) of the magnetization recovering to Mø. Not all of the energy given off is detectable as an RF pulse. Some of the energy goes to heating up the surrounding tissue, referred to as the lattice. In a global, or rather, universal sense, this system can be divided into the spins, and the rest of the universe, or a very large lattice. This type of spin-lattice interaction is the result of the excited system returning to thermal equilibrium. 3. The time course whereby the system returns to thermal equilibrium, or Mz goes to Mø, is mathematically described by an exponential curve. This recovery rate is characterized by the time constant T1, which is unique to every tissue. As will be discussed in detail later, this uniqueness in Mz recovery rates is what enables MRI to differentiate between different types of tissue. At a time t=T1 after the excitation pulse, 63.2% of the magnetization has recovered alignment with Bø. 3b) Now explain Magnetic Resonance Imaging. Every proton has its own spinning, named precession, which causes an electric field. This electric field gives the protons a direction. The MRI-scanner contains two big magnets. These magnets create a magnetic field in which the protons align with the magnetic field or align against the magnetic field. So the protons spin again, but this is a different type of spinning. A little more protons align with the magnetic field than against the magnetic field. The MRI scanner sends out random radio frequencies because the radio wave frequency has to be exact the same as the precession of the protons for the protons to absorb the energy of the radio waves. The absorption causes a turn of the protons by 90-180 degrees. The longer the radio waves are applied, the more the protons get tilted. When the radio waves stop, the protons emit the energy that was absorbed and the protons return to their original state. The signals that return are different in each kind of tissue. The emitted energy can then be measured. In this way the density of the protons can be determined and an image can be created. 3b) What is the purpose of the gradient of the magnetic field? If you have a homogenous magnetic field, the strength of the field is everywhere the same. This has as result that every proton that has the same precession will absorb the same radio frequency and will emit out the same signal. In this way you can’t locate where the signals come from. An inhomogenous magnetic field differs in strength of the field. The gradient results in differences in precession of the protons. Because of this, the protons absorb also different radio frequencies and emit different signals. In this way different signals return to the MRI and then it is possible to locate where the signals came from. 3b) What is fMRI? Functional MRI is used for neuroimaging. Hemodynamic responses on neural activity during tasks are measured. The fMRI is a comparison of two MRI scans made during a task and during rest. In these scans the consumption of oxygen is measured, also known as the BOLD technique. 3b) What is the BOLD technique? BOLD stands for Blood-oxygen-level dependent signals. The BOLD technique is used to determine where the neural activity is. Oxygenated hemoglobin has a lower magnetic field than deoxygenated hemoglobin. This is because of the own magnetic field Fe had when it hasn’t bound oxygen. 3b) What is angioMRI? AngioMRI makes use of MRI scans to make images of the arteries, to investigate if there are any stenosis or aneurysms in the vessels or arteries. There are two different methods, Flow dependent angiography and Flow independent angiography. Flow dependent angiography uses signals from moving blood vs static tissue. Flow independent angiography uses contrast agents like iodine, which makes the blood vessels brown. X-ray tomography 4a) What are X-rays? How are they generated. X-ray computed technology is a technology based on tomography that uses x-rays to produce virtual slices (layers) that produce an image of specific areas of the scanned object. X-ray is electromagnetic radiation and uses a wavelength between 0.01 and 10 nanometres. There are different kinds of X-rays that are used for different kinds of tissues. X-ray was discovered by Wilhelm Röntgen in 1895. Tomography means imaging by sections. In computed tomography, or CT, X-rays are used to image. X-ray computed tomography is used in X-ray Crystallography, Mammography, Medical CT and Airport Security. All use different wavelengths and photon energy’s. The wavelength used is in between visible light and gamma rays. The different wavelengths will show different body tissues. For example, lower wavelengths are used for mammography, higher wavelengths are used in detecting machines in airports. 4a) What equipment is needed? X-rays are created by a difference in voltage between the anode and the cathode. The electrons want to move from the cathode (which is the negative part) and the anode (the positive part). This causes a electron flow towards the anode, which is called an electron beam. The electrons bump out an electron from the anode. When the same (or another) electron moves back, it will send out photons. These are the xrays. Every ray that hits the screen, will turn black. These rays are not absorbed by the body, for instance. The anode’s material is very important, because different anodes will get different graphs. There is an oil bath in the devise, which is used to cool down the whole devise. The motor turns around so the generated heat is distributed. A vacuum tube is used so water or air won’t disrupt the image. The filter in the X-ray machine is necessary for creating the desired wavelength. In brake radiation, a passing electron is lowered in energy by being slowed down by the atom core and hereby energy is released as radiation waves. In collision a colliding electron bumps an electron from a higher orbit to a lower orbit. This creates radiation waves as well. 4b) Describe recent progress of XCT. The first scanner was called a single-slice axial CT-scanner. It had a single emitter and a single detector. It made single images over a single axis. A disadvantage was that it took 30 minutes to make a scan. Another disadvantage is that the patient was exposed to the radiation for 30 minutes. The last disadvantage is that you had to turn the machine to get a full image of the body. The second generation had a fan shape beam and had multiple detectors. It had still a single axis, so you still had to adjust it. An advantage was that it only took 10 minutes to make a scan. Slip-ring technology: there are moveable emitters and detectors in a ring, so you get a helical view. This creates more information. There is also overlapping between slices. The distance between the slices is a pitch. When there is a low pitch, there is a higher overlap and a longer scanning time. It takes longer to scan the whole distance you need. With a high pitch you can scan a longer area of the body in a shorter time. Nowadays there is a multi-slice CT-scan. There are 4+ detectors and multiple rays. There is still a helical view and more overlapping ribbons. This increases a better view. The scan is quicker and you can scan a larger part of the body. The scan is therefore safer, because the amount of radiation that the patient is exposed to is much lower. There is also a possibility of 3D images. 4b) Explain the principles of the recently introduced dark-field method. The dark-field method: improving the contrast of a picture by only using scattered light. There are two gratings: the second one (G2) is placed behind the first one (G1), so that the holes of the first one are blocked. This creates a dark field, because no light can pass the second grating. When you put an object in front of the first grating, it creates scattering. Light gets through the two gratings, so that it can be detected. The background is not scattered. When light passes the first grating, it will also result in diffraction, which makes extra noise. Diffraction patterns repeat itself, after a certain distance. This is the Talbot effect. If you place the second grating at that distance, the diffracted rays will be blocked. Furthermore, the use of homogeneous light reduces diffraction. This is created by G0. The advantage of the method is that you can create a higher contrast. The disadvantage of the method is that the patient is exposed to a high dose of radiation 4b) Give an example of the improvement of image quality. On the picture you can see an image of a chicken wing. The left image is a conventional transmission image and the right image a dark-field one. Scattering of the chicken bones creates a strong signal in the dark-field image. Even though you can already see the bones in the transmission image, the dark-field image provides an enhanced contrast. This enhanced contrast can help doctors see micro fractures in bones, which they wouldn’t be able to see on a conventional transmission. The image on the right however requires a high dose of radiation. This means it might not be as good as it seems. Electron microscopy 5a) Explain what electron microscopy is. Electron microscopy is a technique that uses a beam of electrons and an electromagnetic field to visualize a specific object. In contrast, a normal light microscope uses beams of photons and glass lenses. Functioning of the microscope consists of a few phases. First an electron gun fires electrons at an object. This beam is focused by electromagnetic fields. The electrons interact with the object, but not every part of the object interacts the same way with the electrons, because every substance differs in the admittance of electrons. After transmission, the electrons are captured by a fluorescent screen. The number of captured electrons gives an indication of the density of the substance. This is what creates the image. 5a) What is the wavelength of an electron? The wavelength of an electron depends on its speed. This is determined by the voltage (Va) that is used for the acceleration of an electron. The formula to calculate the ℎ wavelength of the electrons is 𝜆 = (Planck’s law). √(2𝑚𝑒𝑉𝑎 ) A commonly used acceleration voltage is 100 kV. At Va = 100 kV, electrons have a wavelength of 0,004 nm. 5a) What is the spatial resolution of an electron microscope? The spatial resolution of an EM is extraordinary high in comparison to a light microscope (0,1 nm versus 1 μm respectively). This is due to the fact that the wavelength of electrons is a lot smaller than the wavelength of visible light. 5a) What are primary electrons, secondary electrons, backscattered electrons, Auger-electrons? - Primary electrons: The electrons that are shot out of the electron gun. - Secondary electrons: When a primary electron pushes an electron out of the orbital of an atom, this pushed-out electron becomes a secondary electron. - Auger electrons: A primary electron pushes an electron out of the core orbital of an atom. A higher energy electron from an outer orbital will take its place. The energy that is generated by this process will accelerate another outer orbital electron. This electron is called the Auger electron. - Backscattered electrons: Electrons from the gun interact with the object in a way that they will scatter back towards the gun. 5a) What is the difference between elastic and non-elastic scattering? - Elastic scattering: An interaction between the electron and the specimen that doesn’t cause energy to be transferred from the electron to the specimen. - Inelastic scattering: An interaction between the electron and the specimen that causes part of the energy or all of the energy of the electron to be transferred to the specimen. Ant Pollen grains 5b) What are the essential differences between Scanning Electron Microscopy and Transmission Electron Microscopy? What causes the appearance of contrasts? Scanning electron microscopy: Electrons are used to get an image of your specimen. The specimen has to be in a vacuum, because molecules in air or water will cause electrons to scatter. The whole specimen gets scanned in a raster pattern and from there you can visualize an image. Primary electrons come from an electron gun, an anode lets those electrons accelerate. Electromagnetic fields and lenses are used to focus the beam in a straight line on the specimen. A condenser lens is used to create a small spot of electrons, which scans the specimen. The size of this spot determines TEM the resolution. SEM detects backscattered SEM electrons, secondary electrons and x-rays. Low energy electrons High energy electrons Backscattered electrons are reflected after collision with the specimen, whereas Scanning beam Fixed beam secondary electrons are emitted by the molecules of the specimen. The amount of Image by: sec. e- and Image by: primary eemitted secondary electrons and their back-scattered evelocity depends on the height and the Resolution max 1 nm Resolution max 0,1 nm slope of the surface. A tilted surface emits more electrons than a flat surface. When 2D (‘3D’) image: surface 2D image more electrons are emitted, the signal will be brighter. X-rays aren’t used for imaging, Contrast: back-scattered Contrast: scattering and but give characteristic chemical einterference information about the emitting atoms. 2D images (look like 3D) are produced of Object between detector Object between condenser surfaces. Contrast caused by: difference in and objective and objective electron scattering by different atoms. Transmission electron microscopy: Transmission Electron Microscopy works with thin slices of specimen (about 100 nm). These can be treated with heavy metals. These slices have to be thin, because many electrons should be able to pass through the specimen. The electrons that pass through reach the base plate. There they react with a material that is able to absorb the electrons and convert it into light. The pattern that has been formed can be seen on the plate. The different structures in the specimen cause the contrast. When electrons hit atoms with a large nucleus, they rebound. Electrons can be repulsed/absorbed in thick/dense areas. The electrons are likely to pass through thinner or less concentrated material. The traversing electrons eventually reach the scintillator plate that converts their energy into light flashes. This differs for each part of the specimen by foretold reasons. The image that is produced greatly corresponds with the differences in reflection and transmission of the specimen. Electromagnetic lenses cause an enlarged projection because they enlarge the amount of the exiting electrons. In order to get a higher contrast or study a specific element, heavy metals are used to stain the specimen. These heavy metals rebound more electrons and therefore the contrast will be higher. There are two types of scattering that affect image formation: elastic (interact with nuclei and don’t change speed/energy but direction) and inelastic (interact with electrons and lose energy/speed and change direction) scattering. 2D images are produced of mainly cellular or other molecular structures. Contrast caused by: elastic and inelastic scattering and interference. 5b) What is Cryo Electron Microscopy? How is it done, for what purpose? Cryo-electron microscopy is a form of transmission electron microscopy that uses rapidly frozen specimen. The specimen is frozen by using liquid nitrogen. After freezing it, the sample is surrounded by amorphous ice and no ice crystals are present. After freezing, the specimen is placed in a cryo-chamber. From outside a knife can be used to fracture the specimen. Finally a thin layer of gold-palladium is ‘sputtered’ on the material, to improve the conductance of the electrons. By freezing the specimen rapidly, vulnerable structures are preserved. The specimen can also be fractured without damaging other structures. Other advantages are that there is less radiation damage and dehydration does not occur. This technique is mostly used to visualize vulnerable biological structures, because it preserves their native structure. 2D image of very vulnerable biological structures. Optical imaging and LASER technology 6a) Explain the principles and transmission pathways of a normal light microscope. Microscope and its parts The microscope lamp is of low voltage but high intensity to generate a lot of light. The light goes through the condenser, which helps to evenly illuminate the sample. This is essential at higher magnifications to give detail and sharpness to the image. The diaphragm helps to control the solid angle of light that comes from the condenser. The objective provides the details we see in the final image. The light then travels to the eyepiece. The image is further magnified here. The cornea and eye-lens are the final part of the transmission pathway. The lenses of persons with normal vision will be relaxed if the person looks into the microscope. Path of light through the microscope The Köhler setup Köhler illumination is a method of specimen illumination used for transmitted en reflected light optical microscopy. Köhler illumination acts to generate an extremely even illumination of the sample and ensures that an image of the illumination source is not visible in the resulting image. Important is to distinguish clearly between the illumination of the object and the adjustment of the microscope. It must be mentioned that the diaphragm of the lamp (and not the lamp philament itself) is focussed on the object. The objective lens creates an image of the object, which is viewed through the eyepiece in such a way that no accomodation of the lens of the eye is needed. Resolving power of the microscope The resolving power of the microscope depends on the limit of resolution caused by the wavelength of light and the numerical aperture of the objective. The gap where light can pass through is indicated in the figure with ‘b’. This is larger than the wavelength of the light passing through. This creates interference patterns. Interference results from differences in optical path between the rays that are emitted at the level of the diaphragm. If the difference in path between two rays equals half a wavelength, the resulting intensity is zero, and if the difference equals the wavelength, the intensity is highest. Careful consideration of optical paths leads to an explanation of the diffraction pattern: If the difference in path between a ray from the center of the diaphragm and a ray at the rim of the diaphragm equals half a wavelength, than for every ray in one half of the diaphragm a ray exists in the other half that has half a wavelength difference in path. Therefore every ray is extinguished by another one, and darkness is the result. In this way the image of one point of the sample exists of a circular spot (called Airy disc) and concentric rings around it. Another nearby point of the sample also generates an airy disc with rings. As soon as the points are so close together that the airy discs partially overlap, the image appears as one blob of light, not two points. This is why diffraction limits resolution The ability of the objective to accept diffracted rays is dependent on the refractive index of the media. In other words this means that not all of the detail of the specimen can be accepted by the objective, some will be captured and will not be visible. 6a) What is the difference between magnification and spatial resolution? Magnification: The Numerical Aperture (N.A.) defines the limit of detail which can be resolved by the lens. It can be calculated with the following formula: N.A. = R.I. sin Ø R.I. is the refractive index of the medium. Ø is the angle the ray makes with the optical axis. Magnification determines what degree of enlargement this detail is presented to the eye. Resolution: Resolution is about details whether magnification is about the degree of enlargement this detail is presented to the eye. Resolution is limited by wavelength of light and the angle of acceptance of rays by the objective. So increasing the resolution will increase the details, but increasing the magnification will not lead to an increase in detail. 6b) Explain the principles of dark field microscopy, provide examples. Dark field microscopy is a bit like bright field microscopy but it uses slightly different principles. A special disk (the Darkfield stop) blocks some light from the light source leaving only an outer ring of light that passes the condenser lens which focuses the light onto the specimen. Some of the light gets scattered by the specimen, this light is captured by the objective lens. The rest of the light, which isn't scattered, is blocked by the objective aperture. The scattered light produces an image on the retina of the eye while the rest of the light is blocked, creating the appearance of an illuminated specimen on a dark background. Dark field microscopy is mostly used for small light-diffracting specimens like bacteria, isolated organelles and single celled organisms. 6b) What is a LASER? LASER stands for Light Amplification by Stimulated Emission of Radiation. It is based on stimulated emission of electromagnetic radiation and the energy states of electrons. 6b) How does it work? There are 3 mechanisms where photons interact with atoms: 1. Absorption: No photons are released when a photon interacts with an atom 2. Spontaneous Emission: There is a random spontaneous release of photons. 3. Stimulated Emission: Two photons are released, in the same direction and phase, when a photon interacts with an atom. LASER uses stimulated emission. The mechanism used in LASER is called population inversion. The principle behind population inversion is to keep electrons in a higher energy state. It can only occur if one of the energy levels has a relatively long half-life. If this is the case, applied light can create a surplus of electrons in that energy state. It turns out that photons that have exactly the same energy as the electrons of the inverted population need to move to their lower energy level can trigger the electron to move. The transition will create an additional photon of the same wavelength, direction and phase. If there are 4 energy levels (E1 to E4), you want to keep the electrons in an energy state between E2-E3. If an electron goes into E2 it will fall to the ground energy level causing spontaneous emission, which you don't want. By keeping the electrons in the stimulated emission zone between E2 and E3, you can double the photons everytime a photon interacts with an atom. LASER also makes use of something called a resonator. A resonator is basically two mirrors aligned perfectly parallel to each other. These mirrors ''bounce'' the photons back and forth creating a wave. These waves have a certain frequency which can exit a tiny hole which creates a laserbeam. The photons that don’t exit the hole keep on bouncing back and forth between the mirrors, so they have a higher chance of stimulating other electrons to go to a lower energy level. This way the beam gets very intense. 6b) What is it for? LASER can be used for all sorts of things: medically, commercially, for the military and microscopy. Think of the laser pointers that are used in lectures, laser cutters or confocal laser scanning microscopy. These are just a few examples of the huge amount of things LASER can be used for. Phase-contrast and Interference microscopy 6c) What is phase-contrast microscopy? Phase contrast microscopy was invented in 1934 by Dutch physicist Frits (Frederik) Zernike (1888 - 1966), who was awarded the only Groninger Nobel Price for it. The main aspect of this invention is to convert phase differences into amplitude variations that can easily be detected. For instance, phase contrast (PC) microscopy can be used to produce high- contrast images of transparent specimens, such as living epithelial cells. It is an interference technique that requires at least partially coherent light to illuminate the specimen (more precisely, partial, longitudinal coherence is required to make PC work). 6c) How does it work? When is it used? The basic principle to make phase changes visible in phase contrast microscopy is to separate the illuminating background light from the specimen scattered light, which make up the foreground details, and to manipulate these differently. The light (green) that passes the condenser annulus is focused on the specimen by the condenser, the bundle of light is now a hollow cone, thanks to the geometry of the annulus. Some of the illuminating light is scattered by the specimen (yellow). The remaining light is unaffected by the specimen and form the background light (red). When observing unstained biological specimen, the scattered light is weak and typically phase shifted by -90° — relative to the background light. This leads to that the foreground (blue vector) and the background (red vector) nearly have the same intensity, resulting in a low image contrast (a). In a phase contrast microscope, the image contrast is improved in two steps. The background light is phase shifted -90° by passing it through a phase shift ring. This is a ring which has a cove in the disc. This cove is less thick so the phase shift comes from the difference in index of refraction (or: speed of light in a medium.) This phase shift eliminates the phase difference between the background and the scattered, leading to an increased intensity difference between foreground and background (b). Some of the scattered light will be phase shifted and dimmed by the rings. However, the background light is affected to a much greater extent, which creates the phase contrast effect. The above describes negative phase contrast. In its positive form, the background light is phase shifted by +90° instead. The background light thus will be 180° out of phase relative to the scattered light. This results in that the scattered light will be subtracted from the background light in (b) to form an image where the foreground is darker than the background, as shown in the figure. Two things happen: Refraction Different index of refraction, different phases and resulting ray will be refracted. Refraction large enough, will not pass through the phase-ring in the phase-plate Diffraction Light interacts with atom, induces vibrations in certain electrons Vibrating electrons emit light Image appears 90 degrees out of phase, cancelling each other out Creates contrast 6d) What is differential interference contrast (DIC) microscopy? Differential interference contrast microscopy (DIC) is an optical microscopy illumination technique used to enhance the contrast in unstained, transparent samples. It works on the principle of interferometry, which is a family of techniques in which waves, usually electromagnetic, are superimposed in order to extract information about the waves. So DIC takes advantage of differences in the light refraction by different parts of living cells and transparent specimens and allows them to become visible during microscopic evaluation. DIC gives a greater depth of focus allowing thicker specimens to be observed under higher magnifications. 6d) How does DIC work? To create contrast polarised light is needed. A differential interference microscopy can create polarised light. This polarised light (45°) travels through a Wollaston prism, causing the beam to be separated into two rays: a sampling ray and a reference ray. These two rays will travel through a condenser to concentrate the light, followed by traveling through the sampling object. The two rays will pass through the sampling object in adjacent points, causing the sampling object to be illuminated by two light sources; one with a polarisation of 0° and one of 90°. The area’s in the sampling object will have different refractive index or thickness, allowing the rays to get different optic paths. This causing a phase shift of one ray compared to another. After passing the sampling object the rays will pass through an objective lens and another time through a Wollaston prism. The last Wollaston prism will combine the two rays to one with a polarisation of 45°. The result is blocked by the 2nd polarisation filter (135°). Only if the two beams through the sample experience different optical path length will the combined beam have a polarisation different from 45° and partially pass through the 2nd polarisation filter. The merging of the rays leads to brightening, darkening and interference of the image. See figure 1 for the schematic set up of a DIC. 6d) When is DIC used? Differential interference contrast microscopy (DIC) has strong advantages in uses involving live and unstained biological specimen. Its resolution and clarity in conditions such as this are unrivaled among standard optical microscopy techniques. It enhances the contrast in unstained, transparent samples. The main limitation of DIC is its requirement for a transparent specimen of fairly similar refractive index to its surroundings. DIC is unsuitable (in biology) for thick specimen, such as tissue slices, and highly pigmented cells. DIC is also unsuitable for most non biological uses because of its dependence on polarisation, which many physical specimen would affect. So in short the advantages are the high resolution, good contrast and it requires no staining. The disadvantages of this technique are that it needs thin transparent samples, it appears in 3-D and gives no effect on birefringent specimens because of polarization. 6d) Provide examples DIC is often compared with phase contrast microscopy, they just show different details of your specimen. Some examples of the difference in details between these two techniques is given figures 2 and 3. links: DIC, rechts: fase contrast microscoop boven: DIC. Onder: fase contrast EEG, MEG, SQUID 7a) Explain the origins of EEG signals. How can EEG signals be used to generate 3D images of the underlying current sources. Provide examples. Bioelectricity Neurons Electroencephalography is a method to record the spontaneous rhythmic electrical activity of a brain through electrodes attached to the scalp. Neurons in the brain are electrically charged, thereby maintaining the brain’s electrical charge or also called bioelectricity. This electric charge is maintained by billions of neurons. Cortical neuron activity generates electrical signals that change in the 10 to 100 millisecond range. Due to this, EEG has an extremely high temporal resolution. Voltage generation Membrane transport proteins pump ions across the neuron membrane and are constantly exchanging ions with the extracellular milieu. When many ions are pushed out of many neurons at the same time, it creates a wave of ions pushed out of neighbouring neurons. This wave of ions can reach the electrodes on the scalp and push or pull electrons on the metal of the electrodes. Metal conducts the push and pull of electrons easily. The difference in push or pull voltages is measured between two electrodes. The voltages over time are measured by a voltmeter and give the EEG. Synchronicity and parallel orientation An individual neuron generates an electric potential that is far too small to be picked up by EEG. Therefore, EEG activity always represents the synchronous activity of thousands or millions of neurons that are orientated parallel relative to each other. If the cells do not have similar spatial orientation, their ions do not align and create waves to be detected. Pyramidal neurons of the cortex are well-aligned and fire together, thereby producing the most EEG signal. Activity from sources deeper inside the brain is more difficult to detect than currents near the skull. EEG wave formation EEG The detected signals are transmitted to an EEG system composed of amplifiers, filters, and paper chart or computer monitor. Each electrode is connected to one input of differential amplifier with one amplifier per pair of electrodes. A reference electrode is connected to the other input of each amplifier. These amplifiers amplify the voltage between the active electrode and the reference. A channel represents voltage signal and is depicted as a waveform in the EEG. The more channels are used, the higher the accuracy of the EEG. Standard set-up The recording is obtained by placing electrodes on the scalp with a conductive gel or paste. Two anatomical landmarks are used to position the electrodes: the nasion and the inion, which are the area between the eyes, just above the bridge of the nose and the lowest point of the skull from the back of the head respectively. In the standard set-up according to the lead system, 19 electrodes are placed uniformly along the scalp. In addition, one or two reference electrodes are required. Wave forms An EEG signal is depicted in a number of graphs, in which the voltage is shown on the vertical axis and the time on the horizontal axis. The shape of the graph gives information about the state of the brain functioning, for example sleeping, excited or relaxed state. Scalp EEG activity shows oscillation at various frequencies. Several of these oscillations have characteristic frequency ranges, spatial distributions and are associated with different state of brain functioning, like waking and the various sleep stages. Artifacts Electrical signals that originate from non-cerebral sources are called artifacts, which are almost always present in the EEG data. The amplitude of artifacts can be quite large relative to the size of amplitude of the cortical signals of interest. Some of the most common types of biological artifacts include eye blinks, eye movements and eye muscle activity. Event-related potential Event-related potential are commonly used in experiments in the field of cognitive neuroscience. A subject is repeatedly exposed to a certain type of stimulus, while at the same time the EEG is created. An average is calculated from the EEG signals, yielding a mean brain response to the stimulus. In other words, it gives a depiction of only the brain activity induced by the stimulus. This way, the response of the brain to different type of stimuli can be compared. Creating a 3D image EEG data can be used to evaluate EEG characteristics. This can be done by spectral analysis and 3D imaging. Spectral analysis focuses on the oscillations that can be observed in EEG signals. It provides more detail concerning frequency over time. EEG topography allows mapping of electrical activity across the surface of the brain. It visualizes frequency over time corresponding to a certain region of the brain, providing spatial information. Spectral analysis and topographical mapping can be used together to create a 3D image of the brain activity. Source localization EEG has a low spatial resolution, because a measured electrical signal can originate from different sources in the brain. In fact, the same signal can be produced by many different possible sources. Therefore, source localization is stated as a problem and a solution is required. It is an inverse problem, as EEG data is used to find the sources. Reverse modelling is used for the reconstruction of the source and aims to give an estimation of the location of the source. The accuracy of this method is never 100%, because more than one distribution of the current sources lead to the same EEG. Hence, more knowledge (for instance about conductivity of the brain) is required to distinguish between the possible solutions of the analysis. 7b) What is MEG? MEG is a functional imaging technique that measures magnetic fields generated by neuronal activity. More specifically, it measures the magnetic fields created by post-synaptic potentials. In this way MEG has a higher temporal resolution than other imaging techniques such as fMRI and PET, which measure fuel consumption. Neuromagnetism is the term that is being used for the magnetic fields that are generated by neuronal activity. Neuromagnetism is created simply because electricity flows through Depiction of the application of the the neurons. By using the right-hand rule it can be right-hand rule to a neuron. The white determined what the direction of the magnetic arrows indicate the direction of the field is (see figure 1). electric current whilst the red arrows depict the direction of the electromagnetic field. 7b) Describe the required equipment for MEG. The magnetic field of the neurons is very small. For this reason special equipment is needed in order to be able to measure them. First of all, it is it necessary for MEG to be executed in a so-called magnetic shielding room (MSR). The walls of this room are covered with alternating layers of aluminum and alloy. In addition, active shielding is used. The active shielding system senses incoming signals from outside the room, and produces signals of compensating voltages to annul as much of the incoming signal as possible. In theory, MEG can be more precise than EEG, because the skull will less distort the magnetic signal than the electric signal that is being measured with EEG. 7b) What is SQUID? SQUID stands for superconductive quantum interference device. In MEG, many SQUIDs are being used in order to take measurements. SQUIDS are able to detect very subtle magnetic fields and are thus convenient for measuring the small electromagnetic fields created by neurons (see figure 2). Two main principles derived from quantum physics are of importance for SQUID: 1) superconductivity and 2) electron tunnelling. Both of these will be discussed in the section below. This image shows a rough division between the strengths of electromagnetic fields of different objects. It is clear that femtotesla under which human brain activity falls is a rather small amount of electromagnetism compared to other objects. Superconductivity: Superconductivity is the process in which the cooling down of some particular metals will lead to an electrical resistance of zero. The metal has to reach a ‘critical temperature’ for this to be able to happen. When the resistance of the metals has reached a value of zero, they are said to be in a superconductive state. When these metals are in this superconductive state, they try to stay there. In order to be able to do this, the metals create a so-called ‘screening current’ or Iscreening. This current causes there to be both a current going down and a current going up. The result of this is that both currents will cancel each other out. This is otherwise known as the Meissner effect. Ultimately this means that there is zero resistance. Electron tunneling: Quantum physics is a probabilistic theory. It states that we can never exactly know where an object is, but merely where it is most likely to be. This can thus also be said for electrons. Whilst classical physics assumes that electrons revolve around the nuclei of atoms in circles, quantum physics states that electrons make a probability wave around the nuclei of atoms and that they do not orbit the nucleus in this classical manner. In 1962, Brian Josephson demonstrated the existence and characteristics of the so-called “tunnelling-effect”. This effect can be produced between two superconductive materials separated by a thin insulating layer. This is called a Josephson junction and is also used in SQUID. The Josephson junction enables the flow of electrons, even in the absence of an external voltage. In SQUID, the electron current flow caused by the Josephson junctions flows around the ring. The magnetic field that is measured by the ring will change this electron flow and this will affect the overall current flow across the SQUID. The exact mechanisms of the SQUID are described further below. 7b) Describe therequired equipment for SQUID. A SQUID is created out of a superconductive ring containing liquid helium (for the cooling down) that has two barriers built-in. These barriers are known as Josephsons junctions and are non- superconductive. In the absence of an external magnetic field the current that will run through the ring will be known as the bias current (Ibias) and will be split in half by Josephsons junctions. So now there is an upper 1⁄2 Ibias and a lower 1⁄2 Ibias. When applyling a small magnetic field (for example from neurons) a screening current Iscreening will be observed. The Icurrent flows in the opposite direction of the upper 1⁄2 Ibias and in the same direction as the lower 1⁄2 Ibias. In this way, the lower half of the ring will now have a total current of 1⁄2 Ibias + Icurrent, whilst the upper half of the ring will have a total current of 1⁄2 Ibias – Icurrent. Eventually, the current in the lower half of the ring will be too big to maintain the superconductivity; the critical current is exceeded. This causes the junction on this side of the ring to momentarily become non-superconductive (resistive) and all the current to flow through the upper side. This also causes the current in the upper half to become too big (the critical current is reached) and for the junction to temporarily become non-conductive (resistive). Now that there is a resistance, it follows from the formula U=I*R that there also must be a voltage across the junctions. The voltage is the signal that will be measured and used for imaging (see also figure 3). This image shows what happens when a small magnetic field is send through the superconductive ring of a SQUID. It is visible that there will arise a current and that it will be split in half by the ring. Furthermore, it is visible that a screening current will arise, that will run in the same direction as the lower half bias current. Lastly, it is shown that a voltage will arise in time. Ultrasonic sound 8a) What is ultrasonic sound? Ultrasonic sound is an oscillating sound pressure wave with a frequency over 20.000 Hz. Humans are capable of hearing the frequency between 20-20.000 Hz. The frequency that is used in diagnostic sonographic scanners is 2-18 MHz. A high frequency has a high spatial resolution and less penetration. A low frequency has low spatial resolution and a high penetration. Advantages of ultrasonic sound: - Free of radiaton risk - Relatively inexpensive - Real time images - It is portable 8a) How is ultrasonic sound generated? Ultrasonic sound makes use of the effect of piezoelectricity: When piezoelectric crystals induce electricity, pressure differences arises and pressure differences induce electricity (see figure 1). This was discovered by Jacques and Pierre Curie. Piezoelectricity effect An ultrasound wave is produced by a piezoelectric transducer, the piezoelectric crystal. When there is an electrical charge through the piezoelectric crystal, the crystal expands. When the opposite electric charge goes through the piezoelectric crystal, the crystal contracts. So to create an ultrasonic sound wave, short electrical pulses are formed. These pulses make the filament vibrate and create an ultrasonic pulse (see figure 2). Generation of ultrasound The velocity of the soundwave is determined by the material it goes through. There can not be a vacuum, because the ultrasound needs molecules to move and creates kinetic energy this way. 8a) How can a beam of ultrasonic sound be created and directed? The creation and direction from ultrasonic sound is made possible by phased array. The PA consists of many transducers that can pulse separately. These transducers are put next to each other and the product of these transducers together is a beam of ultrasonic sound. The beam can be directed by the order of activating these transducers (controlled by a computer). In figure 3 there is an example. If you activate the transducers from the right to the left, the direction of the beam will be to the left. So the angle of the ultrasonic sound depends on the timing of the pulses. Phased array Beams of ultrasonic sound are used for medical ultrasonography. If you use multiple probe elements, it is better steerable and it’s tightly focused. A disadvantage of PA is that it is more complex and expensive compared to single-element ultrasonic inspection. 8b) How is the backscattered sound detected? Ultrasound is a wave with a higher frequency than the upper limit of the human hearing range. Ultrasound can be used for medical imaging. Tissue reflects the sound. Variation in density of the different tissues leads to different reflections. A higher dense tissue reflects more of the ultrasonic sound, whereas a lower dense tissue absorbs more and thus reflects less. Piezoelectric crystals detect the backscattered sound. Those crystals generate an electric current when they are compressed (e.g. by a sound wave). 8b) How to generate a 2D image? To create a 2D image the computer calculates the different amounts of reflection from the ultrasonic sound. High reflectivity (sound wave that reflects on bone) is white in the image, low reflectivity is grey (muscle and no reflection is black (water). By calculating the difference in time between different incoming echoes you can measure the distance of the tissue. A bigger difference means the tissue is farther away from the receiver. In the 2D image the farthest tissue is displayed on the bottom and the closest on top. 8b) How can one reconstruct a 3D image from 2D images? By comparing the contour of different 2D images the computer can make clear which contours belong to the same structure. If the contours of two different images are very close together, the surface between the two contours is proceeding towards the “camera”. If they are farther apart the surface is more perpendicular. By using 3D imaging techniques discussed in the first lecture (e.g. making some parts darker than others) you can suggest 3D. 8b) Provide examples. 3D images are sometimes used for early detection of tumours. Most of all parents are curious of what their baby might look like, and a 3D image makes it a lot easier to imagine compared to a 2D image that also shows the internal organs. What is Doppler ultrasound? To be familiar with Doppler ultrasound we must understand the principles of the Doppler effect. For example, the sirens of an ambulance sound different when it drives toward you compared to when it moves away from your location. In ultrasound something similar happens when you focus on the blood flow of arteries and veins. Because the current either moves toward- or away from the sender/receiver, the echo’s frequency changes. When the blood flows towards the receiver the frequency increases and it decreases when it moves away from the probe. The frequency itself depends on the speed of blood flow. By using software that measures the differences in frequencies of the echoes we can determine the speed of blood flow, this is Doppler ultrasound. This technique is used for example by cardiovascular surgeons if they wish to operate a patient and they need to localize a clot. Atomic force microscopy 8c) Describe the composition of an AFM. 1) Cantilever 2) Tip 3) Sample 4) Laser 5) Photodiodes 6) Piezoelectric tube 8c) What are the principles of AFM? Atomic force microscopy makes use of a cantilever with a sharp tip. This tip is usually made out of silicon and its radius is in the order of nanometers. To scan the surface of certain materials, the cantilever is brought close to this material. This is done with a piezoelectric positioning element. Being this close to the molecules of the sample, a lot of forces come in play. Some forces attract the cantilever and some repulse the cantilever. Depending on the material, these forces can be mechanical contact forces, Van der Waals forces, capillary forces, chemical bonding, electrostatic forces and magnetic forces. The change in height due to changes in force are measured by a laser which is pointed onto the cantilever which then deflects in different ways due to these changes. The laser is deflected from the cantilever into a detector which consists out of an array of photodiodes, this data is being used to construct the final image of the sample. There are multiple methods to scan a surface with AFM: the contact mode, the noncontact mode and the tapping mode. With the contact mode the tip scans the surface by coming in close contact with the surface. This is also the common mode used in AFM. While being dragged across the surface, the characteristics of the surface are measured by deflections of the cantilever. The contact mode can also be used to test the thickness, adhesiveness and strength of certain molecules on the surface, it can even be used to test the strength of viruses. However this mode can damage the material by destroying molecular bonds. The non-contact mode makes use of oscillation of the cantilever. Van der Waals is the strongest force that influences the tip. This changes the amplitude of oscillation of the cantilever, which can be measured, and the data can be used to create an image. The tapping mode is used to scan the material without creating potential destructive forces by dragging the tip over the surface (contact mode). Again, oscillation is used with this method, but with a higher amplitude than the non-contact mode. The closer the cantilever tip gets to the surface, the smaller the amplitude gets. Due to this decrease in amplitude, the piezoelectric positioning element tries to maintain the normal amplitude by moving the sample up or down via a feedback mechanism. These changes in amplitude are measured by the deflection of the laser and can be translated into an image. 8c) What are Van der Waals forces? Van der Waals forces are forces that attract and repulse atoms, molecules and surfaces. These forces are different from normal covalent bonds or normal ionic interactions, Van der Waals forces work via electrostatic attraction and repulsion of atoms. They attract when they are far apart, but they repulse each other when they are very close to each other. 8d) Explain image formation with AFM in contact mode (constant force) In this AFM mode the tip of the cantilever scans the sample while it is in close contact with the surface of the sample. This is the common mode used in atomic force microscopy. A force is set on the tip by pushing the cantilever against the sample surface (with a piezoelectric positioning element). This repulsive force has a mean value of 10-9 N. The deflection of the cantilever is sensed and compared in a feedback amplifier. If the measured deflection is different from the desired value the feedback amplifier applies a voltage to the piezoelectric positioning element to raise or lower the sample and restore the desired value of deflection. Sample surfaces are covered by a layer of adsorbed gasses consisting of (primarily) water vapor and nitrogen. When the probe touches this layer, the cantilever is pulled by the surface tension toward the sample surface. When the tip is too close to the surface attractive forces can strongly pull the tip of the cantilever into the surface. To avoid this, contact mode of AFM is done at a depth where the overall force is repulsive but the tip is still in firm ‘contact’ with the solid surface below the adsorbed layers. In contact mode AFM the tip of the cantilever is dragged across the surface of the sample. Contours of the surface are measured by the direct deflection of the cantilever, or (more commonly) by using the feedback signal which is required to keep the cantilever at a constant position. The probe moves in ‘x-direction’ (left to right) and scans the surface in the ‘y-direction’ (front to back). When a detail on the surface appears, the tip moves in ‘z-direction’ (up). This is measured and a computer program will produce with these measurements a 3D image. 8d) Explain image formation with AFM in oscillation (tapping mode) In this AFM mode the tip of the cantilever is not in contact with the surface of the sample, but is oscillating up and down over the surface. The constant frequency of these oscillations is determined by a piezoelectric element in the tip holder. This frequency is influenced by different forces when the AFM tip is moving over the surface at a small distance, like: Van der Waals forces, dipole-dipole interactions, electrostatic forces, etc. Through these forces the amplitude or frequency of the tip is muted. The closer the tip approaches the sample, the more the amplitude will decrease. Just like in the contact mode, a detector registers differences in the amplitude. This detector receives signals from (usually two) photodiodes, which collect the laser beam that is reflected from a laser light on the surface of the cantilever. When the amplitude of the AFM tip is changed by the surface of a sample, the reflection of the laser beam on the cantilever will be changed which causes another distributions of laser light on the two photodiodes. This change in distribution is detected by a detector and is proportional to changes in the sample surface. The advantage of the tapping mode over the contact mode is that the surface of a sample is not damage by the tip. A limitation is that data obtained by the tapping is more difficult to interpret because surface data is indirect measured by forces. 8d) What can be measured using these techniques? The mechanical properties can be measured in the contact mode, by pushing with a certain force on the surface of the cell. With this the breaking point of the cell membrane can be measured. In either the contact mode or the tapping mode the cell surface can be imaged. Also crystal structures can be measured of molecules such as gold. A huge advantage of this imaging mechanism is, little preparation of the sample is necessary. The measurements can be done in physiological conditions, whereas EM has to be done in vacuum. 8d) Examples of AFM images The image on the left shows the surface of the bacterium S. Oneidensis. The image is in real life approximately 5 µm. The little wires between the red bacteria are nano-wires, produced by S. Oneidensis. It is imaged using an AMF in noncontact mode. On the right is an AMF image of the surface of cerium oxide. As you can see using the scale, this image displays the true atomic structure. It is imaged using a conventional atomic force microscope in non-contact mode. Luciferase, Brainbow mouse 9a) Explain how luciferase tagging/labelling works Luciferase is an enzyme, present in animals like the Aurelia aurita (moon jellyfish) and the Photinus pyralis (North American firefly). This enzyme has different functions in these animals. It is used to attract mates, to lure prey or for protection. Luciferase catalyzes the Luciferin-reaction. The following image shows the reaction. The reaction is catalyzed by firefly Luciferase (luciferase derived from the firefly) and takes place in two steps. In the first step, Luciferin and ATP react to in luciferyl adenylate and PPi. The luciferyl adenylate reacts with oxygen, into oxyluciferin in an electronically excited state (carbon oxide, AMP and a hydrogen ion are produced). A photon is emitted as oxyluciferin returns to the ground state. So there is light emission. The color of light that is emitted depends on the type of Luciferin that is used. This chemical reaction is useful for observation of biological processes. It is possible to determine the expression levels of a gene of interest in different kind of cells during three steps. Firstly, luciferase needs to be expressed in the novel host. This is done by transduction. Transduction is the process in which DNA is transferred from one bacterium to another by a virus. Secondly, Luciferin is inserted into the host cells/tissue by a dietary supplement. Finally, the experiment is normalized with the use of a control gene. This final part needs to be done because the transduction of Luciferin is less than 100%. 9a) Can Luciferase and Fluorescent protein tagging/labelling be used only qualitatively, or also quantitatively? Luciferase and Fluorescent protein tagging can be used qualitatively and quantitatively. With quantitative use we can observe a few characteristics: - Gene expression levels - Protein concentration - ATP assays When Luciferase is overexpressed and it becomes abundant in the cell, the ATP becomes the limiting factor. In qualitative use of luciferase and fluorescent protein tagging, the presence or absence of a protein can be observed. The difference between Luciferase and Fluorescent is that Fluorescence absorbs light and when needed it emits the same light again. Luciferase produces it’s own photons out of Luciferin, ATP and oxygen. Because of this, controlling the input controls the output of a Fluorescent protein. When you compare the two, the Luciferase tagging/labelling is better for quantitative observations. For qualitative observations the Fluorescent protein tagging/labelling is favourable. 9a) Give some luciferase examples and practical use If Luciferase is transcribed together with a protein of interest, gene expression and protein concentration could be measured. Another quantitative use of Luciferase is ATP assay in vitro. Luciferase can act as an ATP sensor protein. For the emission of light, Luciferase needs ATP. As long as there is ATP, there will be light emitted. A qualitative use of Luciferase, the presence or absence of a protein can be detected. Luciferase could therefore be used to image cells or subcellular structures. Because Luciferase yield a low signal, it is more appropriate to use Fluorescence as a method for qualitative measurements. 9b) What is a brainbow mouse? How is it created? De Brainbow mouse is een benaming voor een muis waarvan de individuele neuronen gekleurd zijn, zodat neuronen van elkaar onderscheiden kunnen worden. De kleuring wordt veroorzaakt door het gebruik van verschillende soorten fluorescente eiwitten (XFP’s). In de brainbow mouse worden er meestal vier verschillende soorten XFP’s gebruikt. Namelijk het Green Fluorescent Protein (GFP), Yellow Fluorescent Protein (YFP), Red Fluorescent Protein (RFP) en Cyan Fluorescent Protein (M-CFP). Genen of eiwitten kunnen met deze fluorescente eiwitten gelabeld worden, waarna de expressie in vivo kan worden aangetoond. De kleuring van de neuronen is gebaseerd op recombineren met behulp van het Cre/LoxP recombinatie systeem. Cre is een gen uit een bacteriofaag dat codeert voor het recombinase enzym. Recombinase zorgt ervoor dat er een inversie of een insertie plaatst vindt op de ‘Lox plaatsten' in het DNA, die coderen voor verschillende kleuren XFP's. Om het Cre/Lox systeem te kunnen gebruiken, moet er eerst een construct met het Cre/lox systeem worden ingebracht in een cel (figuur 1). Recombinase zal vervolgens willekeurig inserties en deleties uitvoeren op de Lox plaatsten. Het resultaat is een willekeurige expressie XFP's die door licht kunnen worden aangeslagen. Bij het absorberen van de juiste golflengte licht, raakt het eiwit in een aangeslagen toestand en na de relaxatie zendt het eiwit langere golflengtes uit, die zichtbaar zijn als oplichtende structuren. De hersenencellen van een brainbow muis bevatten meerdere constructen, waardoor cellen verschillende kleuren tegelijk uitzenden. In totaal kunnen er ongeveer 100 verschillende soorten kleuren ontstaan. There also is another brainbow constructs which contains an inversed RFP and noninversed CFP, which can flip under the control of the CRE/loxP system, creating either one of the two coulors (red or blue). Another construct is the multiple insertion of the brainbow construct, which will create not 2 or 3 colors, but ~100 colour combinations. All of these constructs are only expressed in the brain because they are under the control of the neuron specific Thy1 promotor. Figuur 1. Voorbeeld van een Cre/Lox construct. De promotor is de promotor van een gen waarin men geïnteresseerd is. De kleuren stellen verschillende XFP's voor. Op de Lox plaatsen kunnen inserties of deleties plaatsvinden. In dit voorbeeld wordt er een GFP afgeschreven en zal de cel een groene kleur krijgen. Voor het bestuderen van neuronen van een brainbow (mouse) kan een confocaal microscoop gebruikt worden. Deze microscoop heeft de voorkeur, omdat het anders lastig is om verschillende kleuren XFP's in één plaatje weer te geven. Een normale fluorescentie microscoop kan maar één soort XFP aanslaan, om een tweede XFP aan te slaan, is er aan andere laser nodig. De confocaal miscoscoop heeft verschillende lasers en kan daardoor makkelijker de verschillende XFP's laten zien (Figuur 2). Figuur 2. Verschillende neuronen, gelabeld en gekleurd door middel van XFP’s. 9b) Provide examples of its use. De Brainbow kleuringsmethode biedt mogelijkheden om de functie en interactie tussen neuronen nader te bestuderen. Zo kunnen interacties tussen individuele neuronen bestudeerd worden, maar kunnen, aan de hand van een neurale map, ook neurologische en psychische stoornissen worden geanalyseerd. Het bestuderen van neuronale connecties en mogelijk deficiënties hierin kunnen bijdragen aan een beter begrip van verschillende hersenziekten. Confocal scanning microscopy 10a) How is a confocal microscope composed? What is the purpose of the LASERs? Confocal scanning microscopy is an imaging technique that makes use of a laser to excite fluorophores in the specimen you want to see. Fluorophores are chemical compounds that can re-emit light after being “excited” with a light beam, in this case a laser. The emitted light always has a higher wavelength (so a lower energy) then the excitation light. 10a) What are the diaphragms (“pinholes”) for? This technique of confocal scanning makes use of pinholes to create the image. Only a small part of the specimen is being collected by the camera each time. The first pinhole creates a very thin beam of light that scans the specimen. The second pinhole makes sure that only the light that is in focus will be measured by the camera. This is to make sure no background haze will be seen in the resulting image, so it will be clearer and better. 10a) What is a dichroic mirror and what is its function? What about the excitation filter and the fluorescence barrier filter? Excitation filter and dichroic mirror The laser passes through the excitation filter and on a mirror, called a dichroic mirror. The excitation filter makes sure that only selected excitation wavelength is let trough when using multiple lasers. Via the dichroic mirror the laser beam is send onto the wanted place on the specimen, but the more important function of this dichroic mirror is that it can reflect light that is shorter than a certain wavelength, but lets light with a higher wavelength pass through. Because the emitted light of the fluorophores in the specimen always has a higher wavelength, the mirror is capable to let only the emitted light pass through. Fluorescence barrier filter The fluorescence barrier filter (emission filter in the figure) can filter out the remaining excitation light from fluorescent light. Hereby, unwanted excitation light such as ultraviolet light is blocked. The emission filter (aka barrier filter or emitter) attenuates all of the light transmitted by the excitation filter and very efficiently transmits any fluorescence emitted by the specimen. This light is always of longer wavelength (more to the red) than the excitation color. Confocal microscopes are used to increase optical resolution and contrast of micrograph by using point illumination and a spatial pinhole to eliminate out of focus light in specimens that are thicker than the focal plane. Examination of thick specimens, intracellulair studies, and in studies where the threedimensional structure of the sample is important. 10a) Explain the principles of fluorescence. Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It also occurs when molecules are excited to higher electronic states by energetic electron bombardment. It is a form of luminescence. The emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. 10a) What is photobleaching? Photobleaching is the photochemical destruction of a fluorophore. Photobleaching may complicate the observation of fluorescent molecules, since they will eventually be destroyed by the light exposure necessary to stimulate them into fluorescence. 10b) Describe the principles of object scanning Traditional fluorescence microscopy tends to enlighten the whole specimen whereas confocal microscopy uses a pinhole as well as point illumination to eliminate any light not coming from within the focal plane from reaching the photodetector/camera, resulting in high resolution and contrast. With this technique thicker specimens can be used. The normal order of components of the microscope is: laser, pinhole, excitation filter, dichromatic mirror, objective, dichromatic mirror, filter, pinhole, detector. Because only one point can be sufficiently scanned due to the properties of point illumination and the pinhole, confocal microscopy is not the fastest of scanning techniques. Scanning multiple sections however allows a form of 3D imaging. 10b) Which parts of the microscope are moved? How? With laser scanning microscopy an image is created by a focused laser beam moving across the sample in a raster pattern. The laser beam is moved across the sample by two moving mirrors. The mirrors are controlled by galvanometer motors. The sample stays in the same place during the creation of the entire image (see figure 1). Figure 1. structure of the microscope 10b) The movement has consequences for some parts of the beams but not for all. Which parts? The exciting LASER beam and thus, the returning LASER beam are redirected by the rotating mirrors. What this means is, that the beams are moved across the specimen, but, as soon as they return via the second mirror, the beams follow the same path as they always do. The exciting beam, reflected by the dichromatic mirror will stay in the same place, as will the returning beam through the dichromatic mirror on the detector. The LASER and the PMT never move. 10b) What is de-scanning? The laser beam is reflected by the dichroic mirror. The beam is then pointed at the specimen and moved across the specimen by the rotating mirrors. When the laser beam has hit the specimen, the wavelength changes. The light that travels back from the specimen will now pass the dichromatic mirror because of it larger wavelength. It will then reach the photomultiplier detector. This process is called de-scanning. 10b) What is the purpose of the photomultiplier? During scanning, the photomultiplier measures the intensity of the fluorescence in combination with the current position of the mirrors. So there will be specific information with specific coordinates. With this information an image can be created. The photomultiplier also enhances the photons obtained from the sample, because only a little light gets past the photocathode. (shown in figure 2) Figure 2. Build-up of the photomultiplier tube 10b) Why would one sometimes use more than one LASER? If you want to detect more than one protein within a sample you can you use multiple LASERS. The proteins need to be colored with different kinds of fluorescent proteins so they can be separated from one another. The used LASERS need to have different wavelengths to excite different colors of light. The use of multiple different blocking filters is needed to filter certain wavelengths of light onto the different photomultiplier detectors. Multiple detectors are needed because the detector makes no distinction between the different wavelengths of light. Figure 3. Result of usage of more than one LASER. In situ hybridisation, Immunocytochemistry, Radioactive labeling, Fluorescence 11a) What is in situ hybridisation, what is its use, provide examples. 11a) What is immunocytochemistry, what is radioactive labeling, what is its use, provide examples.