Medical Device Manufacturing - November 2011
advertisement

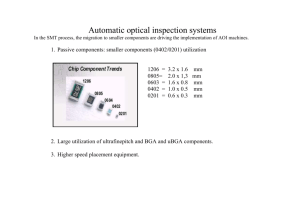
Medical Device Manufacturing – A Look into Good Manufacturing Practices The ability to manufacture medical devices with consistent quality is predicated on a company having well documented and implemented manufacturing processes from incoming inspection until the final product is shipped through the authorized channel delivery system. In this article we will look at the general function of manufacturing disciplines and strategies companies should embrace to ensure that the medical devices they manufacture performs to the exact specifications set for in their product claims. We will look more specifically at electronic manufacturing as it pertains to medical devices, although many of the principals and practices discussed carry over to other manufacturing disciplines. It has been said that your manufacturing outputs are only as good as the quality of your inputs, in this case your raw materials. Companies in the medical device industry have developed sophisticated incoming inspection practices to verify that the components and materials they receive meet the specification set forth in their master bill of material (BOM). In the case of electronic manufacturing, verification of manufacturer, part number, electrical characteristics, tolerances and operating temperature need to be confirmed before the components are brought into inventory. Lot date codes and identification numbers are captured at this point as part of the traceability requirements that we will discuss later. Custom fabricated components that are subcomponents to the final device must also go through such rigor particularly as it pertains to custom plastic housings, bare printed circuit boards and custom fabricated metals. Certificates of compliance (COC) are common for devices that fall into higher risk categories with higher liabilities to the manufacturer. The purpose of requiring COC’s is to document compliance of raw materials, specifications, processes and tolerances for purposes of traceability. Devices that are intended for surgical implant or to support or sustain life are required to establish and maintain procedures for identifying with a control number each unit, lot, and batch of finished devices and components. This enables the manufacturer to perform root cause analysis on any product short coming and isolate the problem. If a particular component or raw material is linked to a failure and is found to deviate from the set forth specification, it can be isolated and other product lots can be traced and isolated to minimize future failures and risk to patients. Any such deviations, recalls or product failures are documented as non conforming with recommended corrective action and this record is maintained in the device history files. A brief discussion of quality systems is warranted here as an introduction to common practices used in manufacturing. FDA 21 Code of Federal Regulation (CFR) Part 820, also known as the Quality System Regulation (QSR) outlines Current Good Manufacturing Practice (CGMP) regulations that govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use. These requirements are meant to ensure that medical devices are safe and effective. Medical device manufacturers undergo FDA inspections to ensure QSR compliance. The FDA has attempted to make these regulations consistent with standards set forth in commercial international standards organizations such as the International Organization for Standards (ISO). ISO 9001 is a comprehensive management system for design and manufacturing. An organization that embraces this system must document all important processes and procedures and demonstrate that efforts are in place for continuous improvement. Another management system that is typically used in conjunction with ISO 9001 is ISO 13485, which requires that management demonstrate that the quality system is in place but does not mandate continuous improvement. One of the most fundamental requirements of ISO 13485 for manufacturing is the inspection and traceability of materials and components. ISO 13485 was published in 2003 and is usually seen as the first step in achieving compliance with European regulatory requirements. A third ISO component recognized today for manufacturers selling product into Europe is ISO 14971, which requires the implementation of a risk management system. The latest revision of this standard was published in 2007 and requires the manufacturer to determine the safety of the medical device during the life cycle of the product. Today’s manufacturing environments have a wide range of sophisticated high speed machinery which requires frequent scheduled inspection to ensure components are being manufactured and assembled to the specified tolerances. In the electronic assembly world, electronic components are placed via robotic pick and place machines on a bare printed circuit board at rates topping 20,000 components per hour which commonly measure .016” x .008”. Ensuring the right components are being placed in the right area in the right orientation with typical tolerances of ± .05 mm requires accurate machine set-up. First articles are run typically with the machines running at a slower rate to make sure the correct components are loaded in the right positions and the proper XY placement coordinates have been loaded into the machine. Routine system checks are performed on automated machinery to minimize down-time. Automated equipment typically has a high capital cost associated with the acquisition and implementation mandating a high utilization rate to justify the expense. Down time also translates into significant loss of production through put impacting revenue. Vision systems help these robots stay in tolerance, but machine parts have normal wear, and a formal scheduled maintenance program should be implemented to adjust, repair or replace wearable components. A company’s GMP documentation will typically have a Standard Operating Procedure (SOP) for the maintenance and calibration of gauges, tooling, instrumentation and equipment used to maintain manufacturing equipment on the manufacturing floor. (See exhibit below Calibration Procedure for Sector Electronics, LLC). High speed 2D & 3D vision systems with resolutions down to 18 µm are becoming a critical asset to manufacturing companies providing early detection of machine errors or process problems early in the assembly process. On an automatic electronic assembly line, vision systems in the solder screening, component placement and final inspection automatically reject sub-assemblies before the full value add is complete. This is a significant cost savings rejecting work in progress and providing for corrective action upstream from the point of reject. This coupled with routine machine maintenance helps minimize defective products and costly rework. Vision systems placed in several strategic locations on the manufacturing assembly line lower assembly cost by detecting early defects, rejecting sub assemblies with minimum value add. In high volume assembly areas, optical comparators are used to compare a golden image with the work in progress assembly on the assembly line. In automated and semi-automated assembly lines, line speed is critical to optimize thru put with yield. As robotic placement speeds increases, the statistical occurrence of errors increases. Most electronic assembly lines will not place an optical vision system between the robotic placement equipment and the solder reflow ovens. Once the printed circuit boards travel through the reflow oven, an optical comparator will look at each board for misplaced and missing components; solder bridging, proper reflow on component leads and other non-conforming processes. These vision systems can scan a complex printed circuit board in less than eighteen seconds but remain a potential choke point for an assembly line. Assembly line balancing becomes critical to minimize choke points and run equipment at speeds that minimize error. Maintenance and calibration of reflow ovens on electronic assembly lines is critical to optimize first pass yield. Thermal profiling of various printed circuit board sizes, layers, copper thickness, laminents and complexity require different thermal ramp profiles to ensure proper reflow of all the solder joints. It is also critical that the cool down period is regulated to ensure a good solder joint. Regular profiling of oven zones is critical to make sure the thermal ramp and cool down rates have been optimized for a particular PCB Assembly. This profile is documented in the device manufacturing file and captures conveyor speed and zone temperatures through a thermal profile device such as a mole. Regulatory agencies, such as IPC, provide will documented visual and x-ray inspection data to validate that the proper processes are in place. Vision systems are very efficient tools as they can visually inspect an entire PCB assembly in fractions of a minute. These maintenance, set-up and auto-feedback systems are tools used to improve first pass yield on complex assemblies. Subassembly and complete system test is essential to comply with ISO 14971 for risk management. Complex systems may require burn in times to ensure proper functionality of the electronics to include thermal, humidity and vibration testing. Other regulatory agencies that are becoming more stringent are FDA, FCC, UL and environmental concerns requiring no lead manufacturing. Although military and medical industries are exempt from the no lead manufacturing requirement today, the time is coming when all manufacturers will need to not only look at their manufacturing environmental impact but also the disposability of their product at the end of life. The new REACH standards in Europe have all of these components which add cost and complexity to those manufacturers selling product in that region. All these factors need to be evaluated as design engineers spec materials and functionality and manufacturing managers’ gear up for production and look at their processes. Below is a typical process control chart used in a CGMP environment. The entire manufacturing process must be flow charted with documentation at key decision points to provision for: non-conformance, corrective action, traceability and feedback loops which are all documented in the device history file. This document should be reviewed at regular intervals to ensure compliance with the mandate of continuous improvement. Sample Flow Chart – Process Control The final step, in most manufacturing processes, is labeling and package control. As the final device is assembled and completes testing, appropriate instruction and warning labels are affixed to the device. Labeling has become a significant component of the FDA inspection. This is part of their mandate of ensuring that medical devices are safe and effective for patient use. Providing clear and concise instructions and labeling are critical last steps before the device is packaged and shipped. Clear and concise labeling is also required on the outside of the device carton with unique identifiers for the device and an associated manufacturing lot. At the end of the day, regulatory controls are getting more arduous and are putting more pressure on manufacturing managers. Having well documented and exercised CGMP’s in place, in the end, makes everyone’s job easier. Routine maintenance and calibration of capital manufacturing equipment greatly increases the probability of shipping high quality, consistent medical devices.