Thank you very much for taking the time to complete this
advertisement
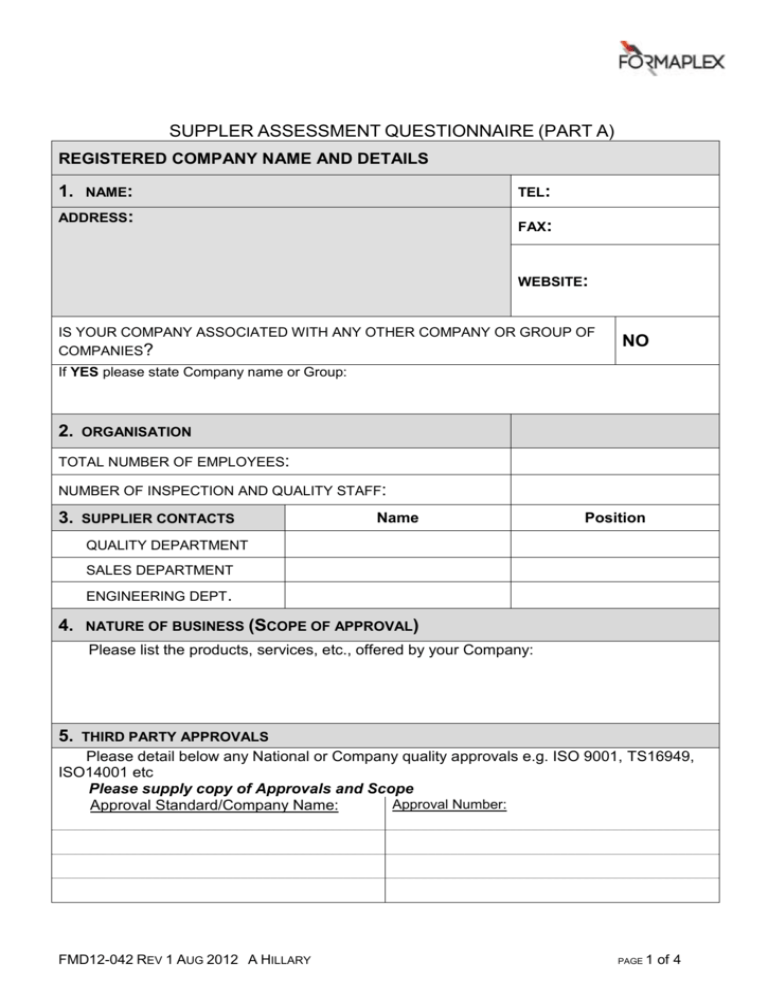
SUPPLER ASSESSMENT QUESTIONNAIRE (PART A) REGISTERED COMPANY NAME AND DETAILS 1. NAME: TEL: ADDRESS: FAX: WEBSITE: IS YOUR COMPANY ASSOCIATED WITH ANY OTHER COMPANY OR GROUP OF COMPANIES? NO If YES please state Company name or Group: 2. ORGANISATION TOTAL NUMBER OF EMPLOYEES: NUMBER OF INSPECTION AND QUALITY STAFF: 3. SUPPLIER CONTACTS Name Position QUALITY DEPARTMENT SALES DEPARTMENT ENGINEERING DEPT. 4. NATURE OF BUSINESS (SCOPE OF APPROVAL) Please list the products, services, etc., offered by your Company: 5. THIRD PARTY APPROVALS Please detail below any National or Company quality approvals e.g. ISO 9001, TS16949, ISO14001 etc Please supply copy of Approvals and Scope Approval Number: Approval Standard/Company Name: FMD12-042 REV 1 AUG 2012 A HILLARY PAGE 1 of 4 6. DOCUMENTED QUALITY SYSTEM Do you have a Quality Manual? Does your quality system show evidence of regular review and update? Do you have a System to communicate change to customers? YES/NO Do you operate Statistical Process Control (SPC)? YES/NO Do you have a Gauge Calibration System? YES/NO Do you document and monitor Internal/External rejects? YES/NO Do you accept the requirements of our document “Supplier Quality Assurance Requirements” Available from www.formaplex .com YES/NO 7. YES/NO YES/NO AUTHORISED SIGNATORIES Please give details below of personnel within your organisation who are authorised to sign release certificates Name 8. Position Signature Stamp ENVIRONMENT Do you have ISO 14001 Approval? YES/NO If NO, do you intend to seek accreditation to ISO 14001? Comments: YES/NO Thank you very much for taking the time to complete this document COMPILED BY: POSITION IN COMPANY: DATE: PLEASE RETURN THE COMPLETED DOCUMENT TOGETHER WITH RELEVANT ATTACHMENTS TO: THE QUALITY DEPARTMENT FORMAPLEX LTD UNIT 1 DAKOTA BUSINESS PARK, DOWNLEY ROAD, HAVANT PO9 2NJ FMD12-042 REV 1 AUG 2012 A HILLARY PAGE 2 of 4 SUPPLIER ASSESSMENT QUESTIONNAIRE (PART B) PLEASE ONLY ANSWER THE QUESTIONS IN PART B IF YOUR COMPANY IS NOT ACCREDITED TO ISO 9001 or TS16949 PLEASE ONLY ANSWER THE FOLLOWING QUESTIONS IF YOUR COMPANY IS NOT ACCREDITED TO ISO 9001. 1. 2. 3. 4. Do you intend to seek accreditation to ISO 9001 or other 3rd party accreditation with a UKAS accredited body? Comments: Do your Systems/Procedures allow for the maintenance of identification/ traceability during processing or manufacture? Do you have a controlled and documented calibration system traceable to National Standards? Do you have a planned and documented internal audit system? YES/NO YES/NO YES/NO YES/NO INCOMING GOODS 5. 6. Do you have a controlled and documented system for inspection of Incoming Goods? Do you maintain a system of rejection/corrective action against the suppliers of incorrect incoming goods? YES/NO YES/NO MANUFACTURING/PROCESS INSPECTION 7. 8. 9. 10. 11. 12. 13. Are all operations checked for conformance at relevant stage inspections and documented? Is Final Inspection performed after completion of all operations/procedures and documented? What form does Inspection take, e.g. 100% or Sampling Plans, etc? Comments: Are records kept of inspections/checks performed so that records are traceable back to the relevant manufactured part? Are all rejected items identified and labelled? Is there a documented system for rejection of items? Is there a documented system for Corrective Action to ensure that the reason for rejection does not recur? YES/NO YES/NO YES/NO YES/NO YES/NO YES/NO SUPPLIERS 14. 15. 16. 17. 18. Do you have a controlled and documented procedure for the use of suppliers? Do you require release certification from your suppliers? Are files of such release documentation maintained? Do you maintain a list of Approved Suppliers? By what methods do you approve suppliers? Comments: FMD12-042 REV 1 AUG 2012 A HILLARY YES/NO YES/NO YES/NO YES/NO PAGE 3 of 4 STORES 19. 20. 21. Is there a Bonded storage area? YES/NO Is there a Quarantine area for non-conforming parts? YES/NO Is access to these areas restricted to authorised personnel? YES/NO COMMERCIAL/QUALITY PLANNING 22. Does your Company have a procedure for Contract Review? YES/NO 23. Does your Company have a procedure for Quality Planning? YES/NO 24. What method is used for recording product history data for traceability purposes, e.g. route cards? 25. How long are such methods kept? Comments: SPECIAL PROCESSES 26. Do you carry out any special processes, e.g. Welding, NDT, Heat Treatment, etc? If YES, please specify: YES/NO 27. Are the relevant procedures in place to control these processes? YES/NO TRAINING 28. 29. Are all your personnel suitably trained to perform their required tasks to the relevant standard? Are individual records kept of relevant training received? YES/NO YES/NO Thank you very much for taking the time to complete this document. COMPILED BY: POSITION IN COMPANY: DATE: PLEASE RETURN THE COMPLETED DOCUMENT TOGETHER WITH RELEVANT ATTACHMENTS TO: THE QUALITY DEPARTMENT FORMAPLEX LTD UNIT 3 DAKOTA BUSINESS PARK, DOWNLEY ROAD, HAVANT PO9 2NJ TEL: +44 (0)2392499276 FAX: +44 (0)2392499277 E-MAIL: tony.hillary@formaplex.com FMD12-042 REV 1 AUG 2012 A HILLARY PAGE 4 of 4