Review 3 - CUNY.edu
advertisement

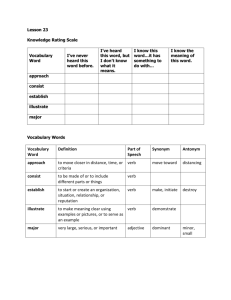
Physical Chemistry II Review Set 3 1. What is this and what are the most important parts of it for this course: 2a. Briefly explain how the interferogram for H-Cl below is generated. What is the structure in the middle called? b. How is the vibrational spectrum of H-Cl obtained form the graph above? Sketch generally what the spectrum would look like. 3. Why is looking at a bright orange stage light more dangerous than watching your leftovers get microwaved? Prove numerically. 4. Explain how Raman vibrational spectroscopy works. Also draw a labeled diagram to illustrate the concept. 5. Explain the concept of an interferometer and its purpose in an FT-IR. Draw a labeled diagram to illustrate the concepts. 6. Explain the difference between fluorescence and phosphorescence. Sketch diagrams to illustrate your explanation. 7. A given monochromatic light has a wavelength 610nm, what are its component colors in the r, g, b? Same question but in x, y, z? 8. What is this and what is its importance to color theory: 9. What wavelength(s) is the color of light at x = 0.4, y = 0.4 in the figure for question 8 composed of? 10. In the 1931 CIE system, x(), y() and z() can be approximated asWyman 2013: Take the Planck distribution to be: where a = 3 × 10-28, b = 0.014 and is in units of nm for all equations. a. Compute the color temperature coordinates X(T), Y(T), Z(T) for a blackbody radiator at T = 2256K between 380nm and 830nm. Hint: Use numerical integration. In Mathematica it is NIntegrate. b. The Gamut coordinates can be computed as: Approximately what color does your answer in a. correspond to? c. Why did we choose the limits of integration to be 380nm to 830nm? Wyman C, Sloan PP and Shirley P. Simple Analytic Approximations to the CIE XYZ Color Matching Functions. J Computer Graphics Techniques, 2013;2(2)1-11. 11. Explain what the Franck-Condon Principle is and sketch a diagram to illustrate your explanation. 12. What is going on here? Tell me everything you know.