2 A + C --> D
Name: ________ KEY ________________ Section: _______________
CHM 152 Exam 1 : Chapters 14 (Kinetics) and 15 (Equilibrium)
Show all work and clearly mark your final answers!
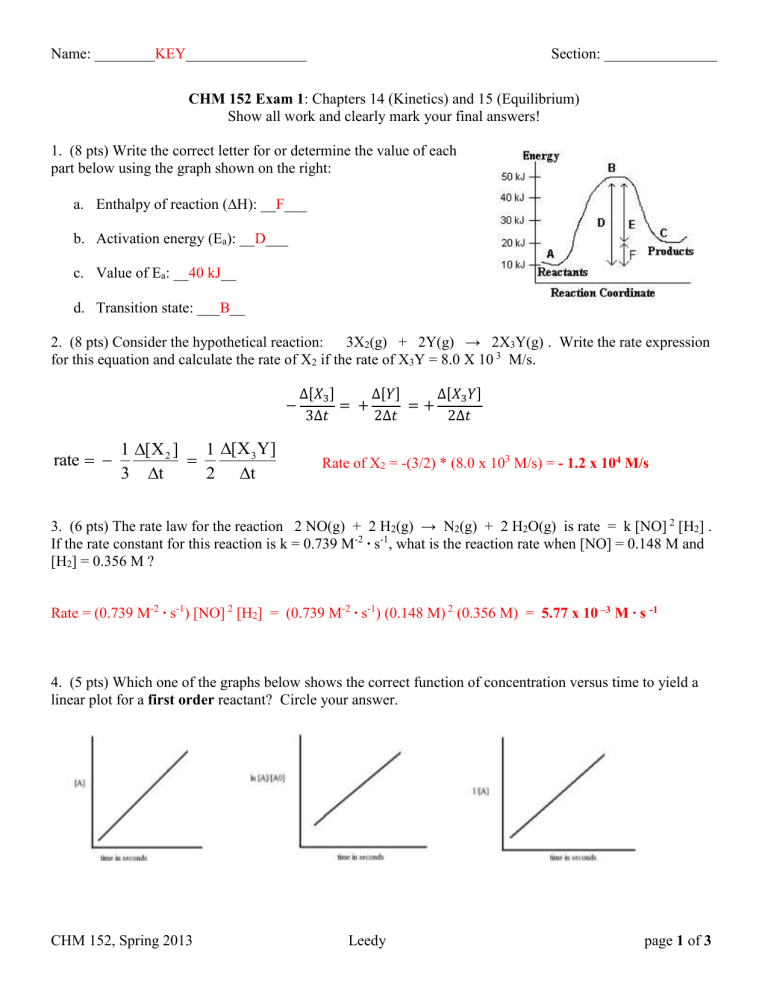
1. (8 pts) Write the correct letter for or determine the value of each part below using the graph shown on the right: a.
Enthalpy of reaction (
H): __ F ___ b.
Activation energy (E a
): __ D ___ c.
Value of E a
: __ 40 kJ __ d.
Transition state: ___ B __
2. (8 pts) Consider the hypothetical reaction: 3X
2
(g) + 2Y(g) → 2X
3
Y(g) . Write the rate expression for this equation and calculate the rate of X
2
if the rate of X
3
Y = 8.0 X 10
3
M/s.
−
∆[𝑋
3
]
3∆𝑡
= +
∆[𝑌]
2∆𝑡
= +
∆[𝑋
3
2∆𝑡
𝑌]
rate
1
3
[ X
t
2
]
1
2
[ X
3
t
Y ]
Rate of X
2
= -(3/2) * (8.0 x 10 3 M/s) = - 1.2 x 10 4 M/s
3. (6 pts) The rate law for the reaction 2 NO(g) + 2 H
If the rate constant for this reaction is k = 0.739 M
-2
2
(g) → N
2
(g) + 2 H
2
O(g) is rate = k [NO]
2
[H
2
] .
∙ s
-1
, what is the reaction rate when [NO] = 0.148 M and
[H
2
] = 0.356 M ?
Rate = (0.739 M
-2 ∙ s
-1
) [NO]
2
[H
2
] = (0.739 M
-2 ∙ s
-1
) (0.148 M)
2
(0.356 M) = 5.77 x 10 –3 M ∙ s -1
4. (5 pts) Which one of the graphs below shows the correct function of concentration versus time to yield a linear plot for a first order reactant? Circle your answer.
CHM 152, Spring 2013 Leedy page 1 of 3
Name: ________ KEY ________________ Section: _______________
5. (8 pts) A second-order reaction has a half-life of 1.20 x 10
2
seconds. If the initial concentration of the reactant is 1.25 M, what is the reactant concentration after 47.0 seconds? t
1
2
1 k [ A ]
0
where t
½
= 1.20x10
2
s; [A]
0
= 1.25 M. k = 1 / (120 s *1.25 M) = 6.66667 x 10
−3
M
-1 ∙ s -1
Now that the value for the 2 nd -order rate constant, k, is calculated, plug this and the given time, 47 seconds, into the integrated rate law for a 2 nd
-order reaction:
1
[ A ] t
= kt
+
1
[ A ]
0
=
(6.6667
´
10
-
3
)(47)
+
1
1.25
=
1.1149
__ A _ 6. (5 pts) All of the following statements are true EXCEPT
And finally, [A] t
= 1 / 1.1149 = 0.898M
a. In a series of stepwise reactions, the rate-determining step is the fast one. b. The rate constant for a reaction can be changed by changing the temperature. c. The rates of most chemical reactions change with time. d. The rate constant is independent of the reactant concentrations.
7. (6 pts) A student gathered data for the reaction H
2
(g) + I
2
(g) ---> 2HI(g) at four different temperatures.
From a known rate law, the student was able to calculate the rate constant at those four different temperatures.
The student plotted (1/T) on the x-axis and (ln k) on the y-axis. The computer-generate best-fit line returned a slope value of -1.95 x 10
4
K and a y-intercept value of +2.739. Calculate the activation energy (in kJ/mol) for this reaction. y = mx + b m = slope m = -(E a
/R)
E a
= - (- 1.95 x 10 4 K) (8.314 J/K mol)
E a
= 1.62123 x 10
5
J/mol = 162 kJ/mol
E a
= m * -R
8. (6 pts) Consider the two-step mechanism shown below for a given reaction.
Step 1: NO (g) + O
2
(g)
NO
3
(g)
Step 2: NO
3
(g) + NO (g)
2NO
2
(g)
What is the overall/net equation? ____ 2 NO(g) + O
2
(g)
2 NO
2
(g) ________
If the overall rate law is: rate = k[NO]
2
[O
2
], which step is the rate-determining step? ___ 2 _______
9. (10 pts) Write equilibrium expressions, K c
and K p
, for the following balanced equations:
N
2
(g) + 2 H
2
(g)
N
2
H
4
(g)
Hg
2
2+
(aq) + 2 Cl
-
(g)
Hg
2
Cl
2
(s)
K
K c c
=
=
[N
2
H
4
] / [N
1 / [Hg
2
2+
2
][H
][Cl
-
]
2
2
]
2
K
K p p
=
=
(P
N2H4
1 / (P
) / (P
Cl-
)
2
N2
)(P
H2
)
2
CHM 152, Spring 2013 Leedy page 2 of 3
Name: ________ KEY ________________ Section: _______________
__ B _ 10. (5 pts) Consider the following equation and equilibrium constant at a given temperature:
N
2
O
4
(g)
2 NO
2
(g), K c
= 0.37. What is the value of K c
for 2 NO
2
(g)
N
2
O
4
(g) at the same temperature? a. 1.6 b. 2.7
c. 9.0x10
2 d. 18 e. 3.2x10
2
__ A _ 11. (5 pts) For the following reaction at 25 o
C, 2 SO
2
(g) + O
2
(g)
⇋
2 SO
3
(g), K c
= 756. What is K p
? a. 30.9
K p
= K c
(RT)
n; b. 179
K p c. 756
= (756)(0.08206*298)
-1 d. 1.85x10
4 e. Not enough information
12. (10 pts) Calculate all equilibrium concentrations of a 0.250 M hydrazoic acid (HN
3
) solution?
HN
3
(aq) + H
2
O(l)
⇋
H
3
O
+
(aq) + N
3
-
(aq), K c
= 1.9 x 10
-5
.
Initial
HN
3
(aq) + H
2
O(l)
⇋
H
3
O
+
(aq) +
0.250 M - 0
N
3
-
(aq)
0
K a
= x
2
/ (0.250 – x)
= 1.9 x 10
-5
Change -x - +x +x
Equilibrium
Equilibrium
0.250 – x
0.248 M
-
-
[HN
3
] = 0.250 – 2.18 x 10 -3 M = 0.248 M x
2.2. x 10
K a
= x 2 / 0.250 = 1.9 x 10 -5 assume x <<0 check x: (2.18 x 10
-3
M / 0.250 M) x 100% = 0.872%
-3 M 2.2. x 10 -3 x 2 x
= 4.75 x 10
M
-6 x = 2.18 x 10 -3 M
Use the reaction 2NO (g) + O
2
(g)
2 NO
2
(g),
H = -374 kJ and K c
= 6.9 x 10
5 at 500 K for # 13 – 16.
13. (2 pts) Is this system reactant-favored or product-favored ? Circle your answer.
14. (6 pts) If a closed container is filled with 0.012 M NO, 0.20 M O
2
, and 0.16 M NO
2
, is the system at equilibrium? Show your work (a simple yes or no answer is NOT sufficient) !
Q c
= [NO
2
]
2
/ ([NO
2
][O
2
]) = (0.16 M)
2
/ ( (0.012 M)
2
(0.20 M) = 889
Q c
< K c
; these concentration values indicate that the system is NOT at equilibrium
15. (4 pts) If it is NOT at equilibrium (based on your answer to #14), in which direction will it need to shift to reach equilibrium? Explain why!
This system needs to shift to the right (
) to reach equilibrium. The ratio of products to reactants will increase until K is reached. The ratio increases when the product concentration increases.
16. (6 pts) which of the following stresses to the system will cause it to shift in the direction indicated in the above question? Circle all that apply. a.
Add [NO] c.
Increase temperature e. Remove [NO
2
] b. Add a catalyst d. Increase volume f. Increase pressure
CHM 152, Spring 2013 Leedy page 3 of 3
Name: ________ KEY ________________ Section: _______________
_______________________________________________________________
Extra Questions:
2. For the reaction: 2 A + 3 B
C + 1/2 D + 2 E; the rate, with respect to A, was experimentally determined to be 0.0185 Mmin -1 . What would the rate be with respect to D?
A: 0.0185 Mmin
-1
B: 0.00925 Mmin
-1
C: 0.00463 Mmin -1
D: 0.037 Mmin
-1
E: 0.074 Mmin
-1
2. The rate law for the reaction 2 NO(g) + 2 H
2
(g) → N
2
(g) + 2 H
2
O(g) is rate = k [NO] 2 [H
2
] . If the rate of this reaction is 5.63 x 10
–2
M ∙ s
-1
when [NO] = 0.242 M and [H
2
] = 1.30 M, what is the reaction rate when [NO] = 0.148 M and [H
2
] = 0.356 M ?
To solve this problem, plug in the values for rate, [NO] , and [H
2
], solving for the rate constant k. After k is calculated, use this value and the new [NO] and [H
2
] values to determine the new rate. rate = k [NO]
2
[H
2
] = 5.63 x 10
–2
M ∙ s
-1
= k (0.242 M)
2
(1.30 M)…… k = 7.39 x 10 – 1 and the new rate is: 7.39 x 10
– 1
[NO]
2
[H
2
] = 7.39 x 10
– 1
(0.148 M)
2
(0.356 M) = 5.77 x 10 –3 M ∙ s -1
3. When two compounds, A and B, are mixed together, they form compound C, by a reaction that’s not well understood. Fortunately, the following rate information was experimentally determined, as shown below:
[A] (mol/L) [B] (mol/L) Rate (mol/L .
sec)
0.050 0.050 4.0 x 10
-3
0.10
0.050
0.050
0.10
8.0 x 10 -3
1.6 x 10
-2 a) Determine the rate law for this reaction. b) Determine the rate constant for this reaction.
The reaction A --> products is second order in A. Initially [A]
0
= 1.00 M; and after 25 mins,
[A] = 0.25 M. What is the rate constant for this reaction?
CHM 152, Spring 2013 Leedy page 4 of 3
Name: ________ KEY ________________ Section: _______________
An
elementary
reaction,
2 A + C --> D
, is second order in A and first order in C. The rate of this reaction is 2.5 x 10
-1
M/s when the concentrations of A, C, and D are all 1.00 mM. What is the rate constant for the reaction?
.
15. Substance A undergoes a first order reaction A → concentration of A in a sample is 1.6 M, what will be the concentration of A after 80 min?
(A) 0.40 M (B) 0.20 M (C) 0.10 M (D) 0.050 M
3. The half-life of 214-Bi is 19.7 min. Starting with 1.00 x 10 -3 grams of 214 Bi, how many grams remain after 59.1 min?
5.00 x 10 -4
2.50 x 10
-4
3.33 x 10
-4
1.25 x 10 -4
10. If the rate constant of a reaction increases by a factor of 5 when the temperature is increased from 22 to
35°C, what is the activation energy (E a
) of the reaction.
A: 93.5 kJ/mol B: 923 J/mol
C: 792 J/mol
D: 53 kJ/mol
E: There is not enough information to answer the question.
8. The reaction mechanism for a reaction is as follows...
Step1
.
Step2
What is the overall reaction?
Identify the molecularity of each step.
Identify all that apply: reactants, products, , [reaction intermediates]
What is the rate law expression for this mechanism?
5: Researchers have proposed the following mechanism for a reaction:
Step 1: NO
2
(g) + F
2
(g)
NO
2
F (g) + F (g) (slow step)
Step 2: F (g) + NO
2
(g) NO
2
F (g) (fast step)
Which of the following statements is/are true? i. F is an intermediate ii. Step 1 is unimolecular
CHM 152, Spring 2013 Leedy page 5 of 3
Name: ________ KEY ________________ Section: _______________ iii. The rate law for the overall reaction would be: Rate = k[NO
2
][F
2
] iv. The overall reaction would be third order
A: i only B: ii only C: i and ii only D: ii and iii E: i and iii
For the following reaction, the rate law is found to be Rate = k[Ce
4+
][Mn
2+
].
One mechanism for this reaction, containing the following elementary steps, is shown below:
1. Ce
4+
+ Mn
2+
---> Ce
3+
+ Mn
3+
2. Ce
4+
+ Mn
3+
---> Ce
3+
+ Mn
4+
3. Tl
+
+ Mn
4+
---> Tl
3+
+ Mn
2+
What is/are the intermediates? _______________ What is the catalyst? _________
What is the overall equation? ________________________________
8. (4 pts) Which of the following equations represents the rate law for the following elementary process: A +
B ----> C + D? a. Rate = k[C][D] b. Rate = k[A] c. Rate = k[A][B]
2 d. Rate = k[A][B] e. Rate = k[B]
6. The reaction X + Y
2M has K c
= 0.89 at 672 K. At equilibrium, a) products predominate substantially b) reactants predominate substantially c) roughly equal molar amounts of products and reactants are present d) only products exist e) only reactants exist
What is the value of Kc for the reaction H
2
(g) + I
2
(g) <===> 2 HI (g) if the equilibrium concentrations for H
2
, I
2
, and HI are 0.15M, 0.033M, and 0.55M respectively?
A: 23 B: 111 C: 0.0090 D: 61.1 E: 0.016
37. A mixture of 2.0 mol of CO(g) and 2.0 mol of H2O(g) was allowed to come to equilibrium in a l L flask at a high temperature. If Kc = 4.0, what is the molar concentration of H2(g) in the equilibrium mixture?
31. The value of the equilibrium constant K for a reaction at equilibrium is altered by
(A) changing the effective concentration of reactants.
(B) changing the effective concentration of products.
(C) changing the temperature.
(D) adding a catalyst.
(E) adding water.
CHM 152, Spring 2013 Leedy page 6 of 3
Name: ________ KEY ________________ Section: _______________
20. Consider the following reaction at equilibrium: 2NH
3
(g)
N
2
(g) + 3H
2
(g),
H = +92.4 kJ. Adding N
2
(g) to the system at equilibrium will __________. a) decrease the concentration of NH (g) at equilibrium b) decrease the concentration of H (g) at equilibrium c) increase the value of the equilibrium constant d) cause the reaction to shift to the right e) remove all of the H (g)
13. (4 pts) For which one of the following reactions are K c and K p
the same? a) H
2
(g) + Cl
2
(g)
2HCl(g) b) 2SO
3
(g)
2SO c) N
2
O
4
(g)
2NO
2
(g) + O
2
(g)
2
(g) d) C(s) + CO
2
(g)
2CO(g) e) 2CO(g)
C(s) + CO
2
(g)
15. For the vapor-phase reaction 2AZ
A
2
+ Z
2
K c
= 16 at 523 K. If 0.030 mol AZ is introduced into an evacuated 1.00 L vessel at 523 K, then at equilibrium [Z
2
] is _____________ M. a) 0.003 b) 0.013 c) 0.017 d) 0.24 e) 0.0052
CHM 152, Spring 2013 Leedy page 7 of 3








