Part 1: Heat of Reaction of a Metal with Acid
advertisement

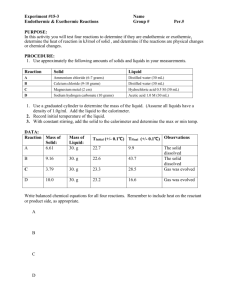
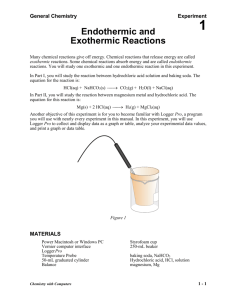
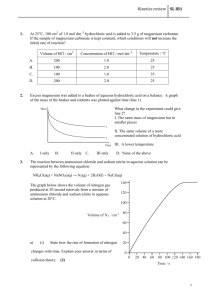
Experiment # 9: Heat and Chemical Reactions Chemical reactions involve heat energy. Sometimes heat is absorbed as in an endothermic reaction. Sometimes heat is released as in an exothermic reaction. In this lab you will carry out three reactions and determine the heat of reaction. You will use the LabQuest and temperature probe to measure the temperature changes. Procedure Set up the LabQuest and the temperature probe to monitor temperature. Be sure to rinse off the probe after placing it into a solution. Part 1: Heat of Reaction of a Metal with Acid 1. Measure out 50.0 ml of hydrochloric acid solution into an empty calorimeter cup that has been massed and record mass of acid. 2. Measure and record the initial temperature of acid solution. 3. Obtain 3.0 cm piece of magnesium metal. Place into acid and stir with probe. Record the final temperature of solution when metal is dissolved and temperature levels off. 4. Rinse the solutions down the sink and rinse out the cup. Part 2: Heat of Reaction of a Solid and Acid 1. Measure 50.0 ml of hydrochloric acid into calorimeter cup. Determine and record initial temperature of solution and mass of acid. 2. Measure 2.00 grams of sodium bicarbonate (NaHCO3). 3. Add Sodium bicarbonate to acid and stir with probe. When the reaction stops and temperature has leveled record final temperature. 4. Rinse out cup and probe. Part 3: Heat of Solution 1. Measure out 2.00 grams of calcium chloride (CaCl2). 2. Place 50.0 ml of water in the calorimeter and record the initial temperature and mass of water. 3. Add the calcium chloride and stir to dissolve. Record the final temperature when all the solid has dissolved. 4. Rinse out the cup. Then repeat the process using 2.00 grams of ammonium chloride (NH4Cl). Be sure to record the initial and final temperatures and to rinse out the cup and rinse off the probe when done. Calculations and Questions Part 1: Heat of Reaction of Acid and Magnesium Metal 1. Write a balanced equation for the reaction of magnesium metal with hydrochloric acid solution. This is a single displacement reaction (what gas was produced?). 2. Calculate the heat change of the hydrochloric acid solution. ( Heat = mass x Temperature change x specific heat). The mass of 50.0 ml of acid solution is about 50.0 grams and the specific heat approximately that of water (4.18J/goC). Convert the heat to kilojoules. 3. How many moles of magnesium metal were reacted? The mass of a 3.0 cm piece is about 0.067 grams. 4. Find the heat of the reaction in kilojoules per mole of magnesium by dividing the heat by the number of moles of magnesium. Was this reaction exothermic or endothermic? Write the amount of heat on the appropriate side of the balanced equation. Part 2: Heat of Reaction of Acid and NaHCO3 1. Write a balanced equation for the reaction of NaHCO3 with hydrochloric acid solution (the products are salt, water and carbon dioxide gas). 2. Calculate the heat change of the acid solution by using the mass (~ 50.0 g), temperature change and specific heat (4.18 joules/goC) as you did in step 2 of Part 1. Convert to kilojoules. 3. Convert the 2.00 grams of NaHCO3 to moles. 4. Determine the heat change of the reaction per moles of NaHCO3. Is this reaction exothermic or endothermic? Place the amount of heat on the appropriate side of the balanced reaction. Part 3: Heat of Solution 1. Determine the heat change in kilojoules of the water when the calcium chloride was dissolved using 50.0 grams of water and the specific heat of water. Was this process exothermic or endothermic? 2. Convert the 2.00 grams of calcium chloride to moles. 3. Determine the heat per mole of calcium chloride. 4. Repeat the calculations for the ammonium chloride to find the heat change per mole of ammonium chloride. Was this process exothermic or endothermic?