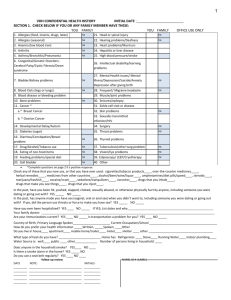
Supplementary Table 3 The prognostic importance of ploidy in
advertisement

Supplementary Table 3 ǀ The prognostic importance of ploidy in patients with early stage ovarian cancer, including BOT Study (year of publication) Dickinson et al. (2012)S29 Lassus et al. (2011)S30 Paulsen et al. (2011)S31 Zeimet et al. (2011)S32 Akeson et al. (2009)S33 Veerman et al. (2009)S34 Verbruggen et al. (2009)S35 Villella et al. (2007)S36 Kulkarni et al. (2007)S37 Kristensen et al. (2003)S38 Flezar et al. (2003)S39 Kimmig et al. (2002)S40 Ozalp et al. (2001)S41 Skirnisdottir et al. (2001)S42 Tropè et al. (2000)S43 Tumour histology FIGO Stage Diploid (%) Follow-up duration End point P value HR Mostly Ia–Ic I–IV 70 98 mo RFS NS NS 440 S BOT, Mu BOT S 57 8 yrs M: P = 0.02 1.79 ICMsuspension ICMsuspension FCM-A 279 S, Mu, E, CC I 62 56 mo U: P <0.01 NA 70 S, Mu I–IV NA NA M: P = 0.003 2.7 153 III 25 81 mo NA 1.4 FCM 135 S, Mu, E, CC, Mx All DFS, OS DFS, CSS RFS, OS OS I–IV NA 53 mo PFS, OS NA FCM 93 57 mo RFS 48 Mostly I–II I 91 ICM-section S BOT, Mu BOT G U: P = 0.012 (PFS) U: P = 0.024 (OS) M: NS NS ? 11 yrs OS U: P = 0.019 NA ICMsuspension ICMsuspension ICMsuspension FCM-A FCM-F 62 S, E, Mu, CC I–II NA 33 mo M: P = 0.04 4.58 284 I 57 13.2 yrs M: P <0.0001 9.1 43 S, Mu, E, CC, Mx S BOT I–III 95 6 yrs DFS, OS RFS, DFS DSS, OS NS NS 62 S, Mu, E, CC Mostly III NA 21.5 mo RFS M: P = 0.03 FCM-F 26 Mostly S I–IV 38 NA U: P <0.05 FCM-A 103 All Ia–IIc NA 74 mo RFS, OS RFS 3.0 In FIGO III NA NS NS FCM-A 162 S, Mu, E, CC, Mx I 45 4 yrs DFS, DSS M: P = 0.003 6.0 Method of ploidy measurement FCM-A Number of patients 70 FCM-A Abbreviations: BOT, borderline ovarian tumour; CC, clear cell; CSS, cancer-specific survival; DFS, disease-free survival; DSS, disease-specific survival; E, endometrioid; FCM, flow cytometry; FCM-A, flow cytometry using archival material; FCM-F, flow cytometry using fresh or frozen material; G, granulosa cell; HR, hazard ratio; ICM, image cytometry; M, multivariate analysis; mo, month; Mu, mucinous; Mx, mixed; NA, not applicable/available; NS, not significant; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; S, serous; U, univariate analysis; yrs, years. S29. S30. S31. Dickinson, P.D., Chan, S.Y. & Sundar, S. The clinical usefulness of DNA aneuploidy in borderline ovarian tumours. Onkologie 35, 607 (2012). Lassus, H., Staff, S., Leminen, A., Isola, J. & Butzow, R. Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol. Oncol. 120, 11-17 (2011). Paulsen, T., Kaern, J. & Trope, C. Improved 5-year disease-free survival for FIGO stage I epithelial ovarian cancer patients without tumor rupture during surgery. Gynecol. Oncol. 122, 83-88 (2011). NS S32. S33. S34. S35. S36. S37. S38. S39. S40. S41. S42. S43. Zeimet, A.G. et al. DNA ploidy, nuclear size, proliferation index and DNA-hypomethylation in ovarian cancer. Gynecol. Oncol. 121, 24-31 (2011). Akeson, M. et al. A population-based 5-year cohort study including all cases of epithelial ovarian cancer in western Sweden: 10-year survival and prognostic factors. Int.J Gynecol.Cancer 19, 116-123 (2009). Veerman, M.M. et al. Clinical value of morphometric and DNA flow cytometric variables as independent predictors of survival in epithelial ovarian carcinoma: a 5-year follow-up study. Int. J. Gynecol. Pathol. 28, 432-441 (2009). Verbruggen, M.B. et al. The prognostic and clinical value of morphometry and DNA cytometry in borderline ovarian tumors: a prospective study. Int. J. Gynecol. Pathol. 28, 3540 (2009). Villella, J. et al. Clinical and pathological predictive factors in women with adult-type granulosa cell tumor of the ovary. Int.J Gynecol.Pathol. 26, 154-159 (2007). Kulkarni, A.A. et al. DNA replication licensing factors and aurora kinases are linked to aneuploidy and clinical outcome in epithelial ovarian carcinoma. Clin. Cancer Res. 13, 61536161 (2007). Kristensen, G.B. et al. Large-scale genomic instability predicts long-term outcome for women with invasive stage I ovarian cancer. Ann.Oncol. 14, 1494-1500 (2003). Flezar, M.S., But, I., Kavalar, R. & Us-Krasovec, M. Flow and image cytometric DNA ploidy, including 5c exceeding cells, of serous borderline malignant ovarian tumors. Correlation with clinicopathologic characteristics. Anal.Quant.Cytol.Histol. 25, 139-145 (2003). Kimmig, R. et al. Multivariate analysis of the prognostic significance of DNA-ploidy and Sphase fraction in ovarian cancer determined by flow cytometry following detection of cytokeratin-labeled tumor cells. Gynecol. Oncol. 84, 21-31 (2002). Ozalp, S., Yalcin, O.T., Gulbas, Z., Tanir, H.M. & Minsin, T. Effect of cellular DNA content on the prognosis of epithelial ovarian cancers. Gynecol. Obstet. Invest. 52, 93-97 (2001). Skirnisdottir, I., Sorbe, B., Karlsson, M. & Seidal, T. Prognostic importance of DNA ploidy and p53 in early stages of epithelial ovarian carcinoma. Int. J. Oncol. 19, 1295-1302 (2001). Trope, C. et al. Randomized study on adjuvant chemotherapy in stage I high-risk ovarian cancer with evaluation of DNA-ploidy as prognostic instrument. Ann.Oncol. 11, 281-288 (2000).