File
advertisement

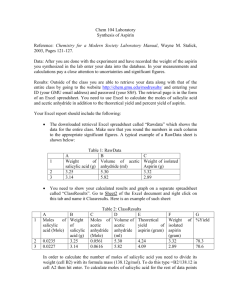
Johnathan Barton CHE111-031 TA: Alex Boehm Lab Partners: Kevin Eversole and Rachel Brakeville 10/23/14 Synthesis & Recrystallization of Aspirin Introduction: If we synthesize aspirin and recrystallize it to produce a more pure sample, then we should be able to determine the theoretical, actual and percent yield. We should also be able to perform a purity test by adding an iron solution to our crystals. If our solution turns into a violet color, then we know that our aspirin is not pure. Aspirin is a commonly used medicine to treat headaches. For many years, salicylic acid (HOC6H4COOH) was used as the common headache medicine. However salicylic acid is much more acidic than aspirin, making it much more displeasing to use. Aspirin can be synthesized by reacting salicylic acid with acidic anhydride according to the following equation in the presence of phosphoric acid as a catalyst: (French et. al, 76). (CH3CO)2O (Acetic Anhydride) + HOC6H4COOH (Salicylic Acid) → CH3CO2C6H4CO2H (Aspirin) + CH3COOH (Acetic Acid) In order to determine how much aspirin is being created, it is important to determine which reagent is the limiting reagent. In a chemical reaction, the limiting reagent is the reagent that is completely used up as the reaction takes its course. Determining the limiting reagent is important when determining the theoretical yield of a reaction. The theoretical yield is how much product we can theoretically create based on the mole ratios from a balanced chemical equation. Using mole ratios given to us by the chemical equation, we can find the theoretical yield using our limiting reagent. After finding the theoretical yield, we can then find the percent yield. The percent yield is the amount of a substance that was yielded from a reaction compared to our theoretical yield. It can be described by the following equation: (French et. al, 77). (Actual yield/Theoretical Yield) x 100 = Percent Yield Before we can determine our percent yield, we must be sure that we are working with a pure substance. To make sure that we have a pure substance, we must filter out any unreacted substances and make sure that we are working with clean glassware. Recrystallization is a way to purify a sample. It contains three steps: 1. Dissolution of the solid to be purified in some hot solvent. 2. Formation of crystals as the solution cools. 3. Recovery of crystals by filtration. Our crystals will be purified in the solution ethanol (French et. al, 77). To test the purity of our crystals, we will be adding an iron solution to our crystals. In salicylic acid, there is a phenol (a functional group present in organic compounds) present. When iron reacts with phenol, the solution will turn a violet color. Phenol is not present in aspirin, so if our sample is pure, then no violet color will be observed. This reaction can be described by the following equation: 6C6H5OH (Phenol) + Fe3+(Iron Solution) → [(Fe(OC6H5)6]3-(Violet Complex) + 6H+ Materials and Procedures: No changes were made to the original procedure from the lab manual. (French et al., 78-80). Discussion: In our experiment, we should be able to synthesize aspirin and purify it by recrystallizing it, filtering out any remaining reactants. We can test the purity of our aspirin by dissolving it in solution and adding an iron solution that will turn our solution a violet color if it has any salicylic acid remaining in it. We recorded that our mass of aspirin that was yielded was .0720 g. Based on our theoretical yield of 2.671 g, our percent yield was significantly smaller; 2.70%. When we added the Iron (III) solution to our aspirin, we observed the solution to be a light violet color, indicating that not all of our salicylic acid was used in the reaction. This means that our aspirin sample was not pure. We believe that we received such a small percent yield because we did not allow enough time for the initial reaction to occur. During our experiment, we observed a large amount of crystals forming in the bottom of the Buchner filter. Our results however did not support our hypothesis. Our actual yield was not close enough to our theoretical yield. Our solution turned a violet color when the iron solution was added, indicating that our sample was not pure. Works Cited 1. French, A., Soult, A., Savas, M., Botha, F., Brock, C., Griffith, C., Hood, D., Kiser, R., O’Connor, P., Plucknett, W., Sands, D., Vance, D., Wagner, W. “Experiment 5- Synthesis of Aspirin” CHE 111 General Chemistry Laboratory Manual. Hayden-McNeil. Plymouth, MI. 2014. 75-80. Web. http://www.chem21labs.com/labfiles/36194_27_05_Exp_FrenchA_6844-2_F14..pdf 23 4 October 2014.