Liver Stinks!
advertisement

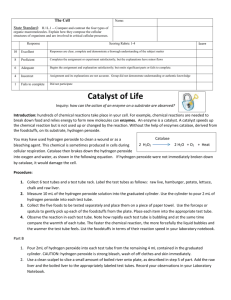
Liver Stinks! Abstract Sometimes science can be really messy or use pretty disgusting ingredients. That is what it takes to understand how the world works, even if the experiment isn't pretty. Do you like chemical reactions that stink and ooze foamy bubbles? Do you think it sounds fun to make a super gross liver smoothie? Then this is the experiment for you! Objective In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Introduction A chemical reaction is when chemical come together and their molecules interact to form new chemicals. Sometimes chemical reactions happen by themselves. These reactions are usually very fast and spontaneous, and give off energy. Other chemical reactions need energy to happen, and without energy proceed very slowly or not at all. These types of chemical reactions can be helped to occur more quickly by using enzymes. Enzymes are made out of protein and they speed up the rate of a chemical reaction by acting as a catalyst. A catalyst provides the necessary environment for the chemical reaction to occur, which speeds up the reaction. Certain catalysts work for certain kinds of reactions, in other words each enzyme has a particular type of reaction that it can activate. Enzymes are proteins, which are molecules that are very large and dynamic. They can be very fussy, and sometimes need to be in certain environments or conditions to work. Some enzymes can be damaged under certain conditions such as heat. A damaged enzyme will no longer work to catalyze a chemical reaction. One source of enzymes is the liver, which needs to break down many substances in the body. Catalase is one enzyme from liver that breaks down harmful hydrogen peroxide into oxygen gas and water. When this chemical reaction occurs, you can see the oxygen gas bubbles escaping and causing the reaction to foam. In this experiment, you will use fresh (or frozen) liver as a source of catalase and investigate how the activity of the enzyme can change under certain conditions. What does catalase do? Under which conditions does it work best? Why do we need catalase in our liver? Terms, Concepts, and Questions to Start Background Research To do this type of experiment you should know what the following terms mean. Have an adult help you search the internet, or take you to your local library to find out more! liver protein enzyme catalase hydrogen peroxide chemical reaction acid base Bibliography Rader, A. (2005). Basics of Biochemistry: Enzymes. Retrieved December 13, 2005, from http://www.chem4kids.com/files/bio_enzymes.html Kids Health: The Nemours Foundation. (n.d.). How the Body Works: Your Liver. Retrieved July 11, 2010, from http://kidshealth.org/kid/htbw/liver.html Find out why you should "Love Your Liver" from the folks at Kids Health: Kids Health: The Nemours Foundation. (n.d.). Your Digestive System. Retrieved June 30, 2010, from http://kidshealth.org/kid/cancer_center/HTBW/digestive_system.html Learn all about free-radicals, nutrition, antioxidants and the body: MadSci Network. (1999, September 27). Where Does Hydrogen Peroxide come from in the Body? Retrieved July 26, 2010, from http://www.madsci.org/posts/archives/sep99/938519528.Bc.r.html Youngston, Robert. 1994. "The Antioxidant Health Plan." Harper Collins Publishers, U.K. Materials and Equipment liver -- either fresh or frozen (Note: If you order fresh liver, be sure to only order a pound or less, not an entire liver. A whole beef liver can weigh 10 lbs!) water hydrogen peroxide (use a new or recently-purchased bottle for best results.) bottle caps medicine dropper salt (optional) boiling pot of water (optional) vinegar (optional) baking soda (optional) Experimental Procedure 1. Cut the liver into little pieces, about 1–2 centimeters in size. You may need your parents help with this. 2. Place the cubes of liver into a blender and add an equal volume of water. 3. Blend on high speed pulsing when necessary, until the liver is smooth, and no chunks are present. Keep the liver extract in the refrigerator. 4. First, test the activity of untreated liver extract by adding a drop of extract to a bottle cap. 5. Add one drop of hydrogen peroxide. You should see a lot of bubbles! 6. You can record this in a data table and it will be your positive control. Use a scale of 0–5, with 5 representing the "most bubbling" and 0 representing "no bubbling." Treatments Bubble Untreated 5 No liver added 0 Boiled Salty Acidic Basic 7. Now take different samples of your liver extract and test different conditions on activity, testing a drop of the treated extract each time with hydrogen peroxide. 8. To test the effect of heat, place a small portion of the extract in the microwave in a microwave-safe bowl. Heat covered on high for 20-35 seconds. Test a drop of the treated extract with hydrogen peroxide and record your results in your data table. 9. To test the effect of freezing, freeze a small portion of the extract in the freezer. When the sample is completely frozen, remove it from the freezer and thaw the sample before testing it for enzyme activity. Test a drop of the treated extract with hydrogen peroxide and record your results in your data table. 10. To test the effect of acid, mix the liver with some household vinegar before testing. Test a drop of the treated extract with hydrogen peroxide and record your results in your data table. 11. To test the effect of a base, add some baking soda to your extract before testing. Test a drop of the treated extract with hydrogen peroxide and record your results in your data table. 12. To test the effect of high salt conditions, mix the extract with some table salt. Test a drop of the treated extract with hydrogen peroxide and record your results in your data table. 13. Try experimenting with any other condition you can imagine! Remember to record all of your results in your data table! Variations How does catalase change activity when different amounts of the enzyme or the hydrogen peroxide are present? The hydrogen peroxide is called the "substrate," and sometimes changing the amount of substrate or enzyme will change how the chemical reaction proceeds. This is called "enzyme kinetics." Conduct your own experiment on enzyme kinetics by changing the amount of liver extract and hydrogen peroxide in your reactions. What did you find out? Another enzyme, called papain, digests proteins and can be extracted from pineapple. One protein that is fun to use for the experiment is Jello, made out of gelatin. Try a similar type of experiment on the activity of papain. The liver contains many enzymes, each important for detoxifying the body. One of the reasons breaking down hydrogen peroxide is important is because if left alone, hydrogen peroxide in the blood can produce free radicals. Free radicals can cause damage to different parts of the body. Test the effect of hydrogen peroxide on different materials around your house, what happens?