Development of a Polystyrene Latex
advertisement

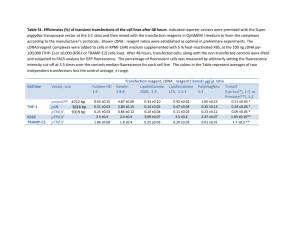
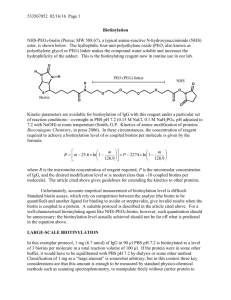
Development of a Polystyrene Latex-Based Reagent for Rheumatoid Factor Detection Marrero G1, Delgado LP1, Carol H2, Ortiz N3, Musacchio A4 and Menéndez T1 1 Centro de Biomateriales (BIOMAT), Universidad de La Habana, Avenida Universidad entre Ronda y G, Plaza de la Revolución, La Habana, Cuba. e-mail:tamara@biomat.uh.cu; 2 Empresa de Productos Biológicos “Carlos J. Finlay”, La Habana, Cuba; 3 Centro Nacional de Reumatología, La Habana, Cuba; 4 Centro de Ingeniería Genética y Biotecnología, La Habana, Cuba. Monodisperse polystyrene latices could be synthesized by emulsifier-free emulsion polymerization methods. The spherical polystyrene particles thus obtained are commonly used as solid support for passive adsorption or covalent coupling of biomolecules with biomedical applications. One of the most common methods for disease diagnostic is polystyrene latex agglutination, which is simple, rapid to perform and results are relatively easy to interpret. Latex agglutination diagnostic reagents are produced by coupling of antigens or antibodies to polystyrene spheres. In the present work we develop a reagent to detect Rheumatoid Factor (RF) which is one of the serologic criteria to diagnose the autoimmune disease Rheumatoid arthritis (RA). The disease affects 1% of population worldwide and causes severe disability and premature mortality. Early diagnosis of disease is of crucial importance since precocious treatment improves overall disease outcome. RF is a group of immunoglobulins IgG, IgM and IgA found in sera of 80% of RA patients. RF targets a specific region of human IgG (Fc region). RF is a prognostic indicator of disease activity and progression and serum RF levels is considered the best laboratory indicator of disease severity. In the present work, spherical, clean polystyrene particles of 480± 80 nm with a Z-potential of -28.32 mV were synthesized. Purified gamma-globulin human blood fraction, used as IgG source, was adsorbed on particles in glycine-buffered saline with a fivefold excess of the immunoglobulins needed to saturate the calculated total surface area of the particles. The reagent was calibrated against the World Health Organization international serum reference preparation and tested with commercial positive and negative RF controls. Reactivity of the developed diagnostic reagent with human sera either positive or negative for RF, was evaluated. The sera were tested both intact and following complement inactivation. When complement-inactivated sera were used, our reagent showed 100% coincidence of results with a commercial diagnostic kit of specificity 80% and sensibility 93%. A rapid, specific and sensible reagent has been developed to detect RF in human sera. The reagent is easy to prepare and at relatively low cost, making it ideal for RF detection in low-income settings.