Handout

10 Equations in Biology Series
I. Definition
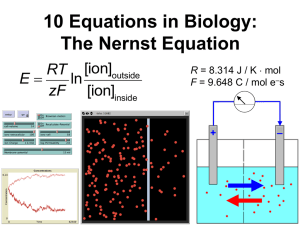
An ion’s equilibrium potential (E) is defined as:
II. Informal Derivation
A. In the chart at right, list up to four specific parameters that you would expect to influence a membrane’s equilibrium potential E for a particular ion. For each parameter, state whether you would expect it to have a positive or a negative effect.
B. In the space below, write a proposed equation for E that includes all of the parameters you listed.
Structure your equation so that each parameter has the desired (+ or –) effect on E.
Seminar #3: The Nernst Equation
Sept. 19, 2012
Parameter
Effect on E
(+ or –)
𝐸 =
III. Nernst Equation: Qualitative Analysis
A. Write the actual Nernst equation in the space at right.
Then define each parameter and state its units. where
B. Dimensional analysis (on board).
E = the equilibrium potential ( in _______ ),
R = the ideal gas constant ( =8.31 J / K mol ),
T = temperature ( in _____ ),
C. In your own words, describe what the ideal gas constant R measures. Then do the same for the Faraday constant F.
z = the ion’s charge ( in mol e – s / mol ions ),
F = the Faraday constant ( =9.65
10 4 C / mol of e – s ), and
[ion] = the ion’s concentration ( in ______ )
D. The ideal gas constant R can be viewed as a conversion factor that relates the physical quantities of energy and temperature. Explain the role that this relationship plays in (i) the ideal gas law PV=nRT and (ii) the Nernst equation.
Page 1 of 2
10 Equations in Biology Series
IV. Nernst Equation: Quantitative Behavior
Seminar #3: The Nernst Equation
Sept. 19, 2012
A. The Nernst equation contains the term ln
[ion] outside . How would the equation’s behavior
[ion] cell be different if this term were instead [ion] outside
− [ion] cell
? Explain.
B. The table below lists three special cases in which the Nernst equation predicts that membrane potential should be either zero or infinite. Complete the table, giving for each case both a clear description of the physical conditions and a biological explanation for why those conditions would yield the predicted behavior.
Value of E Physical description Biological explanation Case
[ion] cell
=
[ion] outside
0
No molecular motion, so no voltage needed to prevent ions from diffusing
Solute has no net charge
(i.e., is not an ion)
V. Application and Interpretation
A. Calculate the equilibrium potential of Na + ions across the membrane of a human skeletal muscle cell. The ion concentration is 15 mM inside the cell and 145 mM in the surrounding extracellular fluid.
B. Calculate the equilibrium potential of Cl – ions across the membrane of a squid giant axon.
The ion concentration is 40 mM inside the cell and 560 mM in the surrounding extracellular fluid (which is isotonic with seawater).
C. For each of the problems above, provide a clear biological interpretation of your result.
This interpretation should include statements about the concentration gradient, the sign of the net cytoplasmic charge needed to maintain that gradient, and plausible mechanisms by which the cell might maintain that charge.
Page 2 of 2