Section 4_1 Notes
advertisement

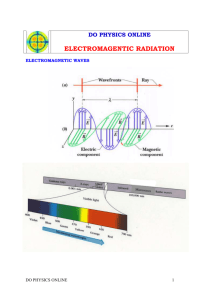
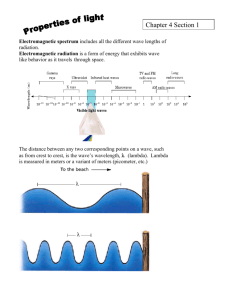
Section 4.1 Notes Key Notes Chemistry Class Ms. Cox The Development of a New Atomic Model Light has characteristics of both particles and waves. The wave description of light – Waves have a repeating or periodic nature. The wavelength (λ) of a wave is the distance between corresponding points on two consecutive waves. Wavelength is measured in meters, centimeters or nanometers. The frequency (ν) of a wave is the number of waves that pass a point on a given amount of time. Frequency is expressed in waves per second, also known as a hertz. Visible light is another type of wave. It is a form of electromagnetic radiation, which is energy that travels through space in the form of a wave. All of the above types of electromagnetic radiation make up the electromagnetic spectrum. All forms of electromagnetic radiation travel at the same speed through a vacuum! This speed is 3.00 x 108 m/s and is called the speed of light. The speed of light slows down a little bit when it passes through matter, such as when light passes through water. The frequency and wavelength of a wave are related to each other by the speed at which the wave travels. For ANY electromagnetic wave, this relationship is written as: Because C is the same for all electromagnetic radiation, the product λν is constant. Therefore, the wavelength of an electromagnetic wave is inversely proportional to its frequency. In the photoelectric effect, a metal gives off electrons when light shines on it. This only happens if the frequency of light is above a certain value. Why? The particle description of light – German physicist Max Planck suggested that a hot object does not emit energy continuously in the form of small waves. He suggested that the object emits energy in small packets called quanta. A quantum of energy is the minimum quantity of energy that can be lost or gained by an atom. The relationship between the frequency, ν, of radiation in 1/s anda quantum of energy, E, in joules is: In 1905, Albert Einstein, expanded on Planck’s by hypothesizing that electromagnetic radiation can behave like a wave AND like a particle. While light can exhibit wavelike properties, it can also be thought of as a stream of particles. Each particle carries a quantum of energy. Einstein called these particles photons. A photon is a particle of electromagnetic radiation having no mass and carrying a quantum of energy. The energy of a photon is given by Planck’s formula for quanta of energy: E=hv. Einstein explained the photoelectric effect by proposing that matter can only absorb a whole number of photons. Matter cannot absorb part of a photon. An electron can only be ejected from an object if one photon is absorbed that has enough energy to knock it loose. Even a large stream of photons striking an object will fail to knock any electrons loose if the individual photons contain too little energy. The formula E=hv relates the minimum energy to a frequency. Because electrons are bound with different force to different metals, the minimum frequency changes for each metal. Electrons exist only in very specific energy states for atoms of each element. When electric current is passed through hydrogen gas in a glass tube, at low pressure, a pinkish light was emitted. The wave theory of light predicted that a continuous spectrum would be emitted. A continuous spectrum is a continuous range of frequencies of electromagnetic radiation. A continuous spectrum of light appears white but the hydrogen gas glowed pinkish and emitted only 4 wavelengths in the visible spectrum. Attempts to explain the emission line spectrum of hydrogen led to the development of the quantum theory of atoms. The theory states that an atom’s energy can only be at specific values, called states. The lowest energy state of an atom is its ground state. Any state that has a higher potential energy than the ground state is an excited state. When an excited atom falls to its ground state or a lower energy excited state, it gives off a photon. The energy of the photon (E=hv) is equal to the difference in energy between the two states. The fact that hydrogen atoms emit only specific frequencies of light suggests that the energy states of a hydrogen atom are fixed. Light can only be emitted with an amount of energy that is the difference between two of hydrogen’s energy levels. Bohr’s model of the hydrogen atom explained electron transition states. According to Bohr’s model, the electron in a hydrogen atom can only circle the nucleus in certain paths or orbits. The orbit the electron is in determines the energy of the atom. The hydrogen atom is in the ground state when the electron is in the orbit closest to the nucleus. The electron cannot go any closer to the nucleus than this orbit. If the electron goes into an orbit that is farther from the nucleus, the atom has more energy. Each successive orbit is a higher energy state. The electron orbits can be compared to the rungs of a ladder. When you stand on the first rung, you have less potential energy than you would if you were standing on any other higher rung. Because you can’t stand between rungs, you can only have amounts of potential energy corresponding to each rung. The electron moves to a higher orbit if it absorbs a photon with energy exactly equal to the amount of energy between the higher orbit and the present orbit. This process is called absorption. When the electron falls to a lower orbit, it emits a photon with the difference in energy between the higher and lower orbits. This process is called emission. Photons emitted by a hydrogen atom can only have energies that represent the differences between its energy levels. This pattern provided convincing evidence that Bohr’s model of the hydrogen atom was correct. This model consisted of an atom with a densely packed nucleus surrounded by electrons in various orbits far from the nucleus. However, Bohr’s model was unable to explain the emission-line spectra of atoms other than hydrogen. Bohr’s model also failed to explain the chemical behavior of atoms.