Group 4 Chemistry - Fulton County Schools
advertisement
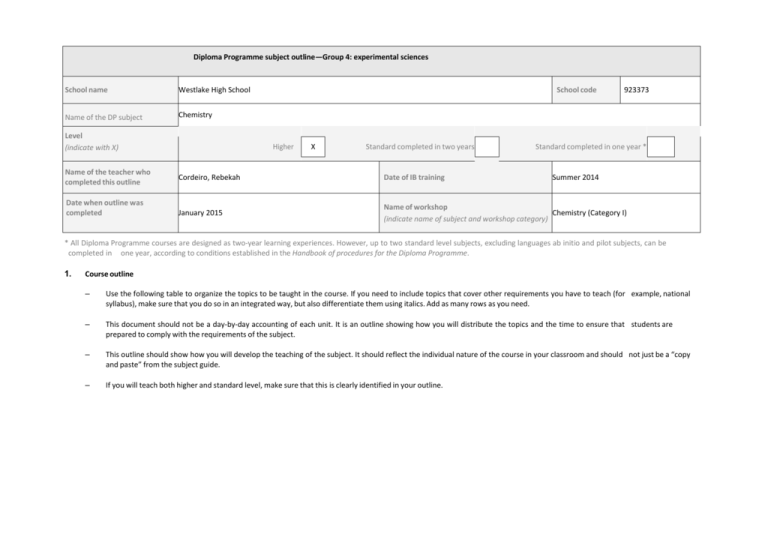
Diploma Programme subject outline—Group 4: experimental sciences School name Westlake High School Name of the DP subject Chemistry Level (indicate with X) Name of the teacher who completed this outline Date when outline was completed School code Higher X Standard completed in two years 923373 Standard completed in one year * Cordeiro, Rebekah Date of IB training Summer 2014 January 2015 Name of workshop Chemistry (Category I) (indicate name of subject and workshop category) * All Diploma Programme courses are designed as two-year learning experiences. However, up to two standard level subjects, excluding languages ab initio and pilot subjects, can be completed in one year, according to conditions established in the Handbook of procedures for the Diploma Programme. 1. Course outline – Use the following table to organize the topics to be taught in the course. If you need to include topics that cover other requirements you have to teach (for example, national syllabus), make sure that you do so in an integrated way, but also differentiate them using italics. Add as many rows as you need. – This document should not be a day-by-day accounting of each unit. It is an outline showing how you will distribute the topics and the time to ensure that students are prepared to comply with the requirements of the subject. – This outline should show how you will develop the teaching of the subject. It should reflect the individual nature of the course in your classroom and should not just be a “copy and paste” from the subject guide. – If you will teach both higher and standard level, make sure that this is clearly identified in your outline. Topic/unit (as identified in the IB subject guide) State the topics/units in the order you are planning to teach them. Year 1 Allocated time Contents One class is 90 In one week there 2-3 are Topic 11: Measurement and data processing (this topic will be practiced throughout the course, but will be taught at the beginning of the course with a real-world project using spectroscopy as a review of pre-IB chemistry) 11.1 Uncertainties and errors in 10 hours measurement and results 11.2 Graphical techniques 11.3 Spectroscopic identification of organic compounds Topic 1: Stoichiometric relationships Topic 9: Redox processes 1.1 Introduction to the particulate nature of 13.5 hours matter and chemical change. 1.2 The mole concept 8 hours 1.3 Reacting masses and volume 9.1 Oxidation and reduction 9.2 Electrochemical cells Topic 2: Atomic structure Topic 12: Atomic structure 2.1 The nuclear atom 2.2 Electron configuration 12.1 Electrons in atoms 6 hours Topic 3: Periodicity Topic 13: The periodic table- the transition metals 3.1 Periodic table 3.2 Periodic trends 13.1 First-row d-block elements 13.2 Colored complexes 6 hours Topic 4 Chemical bonding and structure Topic 14: Chemical bonding and structure 4.1 Ionic bonding and structure 13.5 hours 4.2 Covalent bonding 4.3 Covalent structures 7 hours 4.4 Intermolecular forces 4.5 Metallic bondng 14.1 Covalent bonding and electron domain and molecular geometries 14.2 Hybridization 2 hours 4 hours minutes. classes. Assessment instruments to be used Resources List the main resources to be used, including information technology if applicable. Formative and summative The textbook resource used in the assessments will be used course will be Tro’s Chemistry: throughout the course to A Molecular Approach, 3rd allow students to reflect on Edition. This textbook is very the content they are visually appealing and the learning and to prepare for microscale to macroscale the IB internal and external application of content seems to assessments. engage students in a way that other textbooks do not. This text All summative tests given also offers an online aspect with in the course will mirror full etext and study area, as well the time restraints of the IB as a way to assign work or external assessment, so quizzes through the site, that students practice masteringchemistry.com. performing in the testing environment. At this time, a formal lab manual has not been decided. Inquiry Formative assessments will labs will be the focus, so a be given in the class with manual that goes along with that feedback to prepare will be chosen. Students will be students for internal asked to have and keep their own assessments. For lab notebooks. laboratory projects that are not to be formally A class set of iPads will be internally assessed, available for this course, so students will practice virtual labs and simulations will writing, researching, be used to reinforce ideas designing, and alongside the in-class labs and communicating analysis of activities. Students will have laboratory work. access to iPads in class for research, data collection, and writing reports. Topic/unit (as identified in the IB subject guide) State the topics/units in the order you are planning to teach them. Year 2 Topic 5: Energetics/thermochemistry Topic 15: Energetics/thermochemistry Contents One class is 9 hours Topic 7: Equilibrium Topic 17: Equilibrium 7.1 Equilbrium 17.1 The equilibrium law 4.5 hours 4 hours Topic 8: Acids and bases Topic 18: Acids and bases 8.1 Theories of acids and bases 8.2 Properties of acids and bases 8.3 The pH scale 8.4 Strong and weak acids and bases 8.5 Acid deposition 18.1 Lewis acids and bases 18.2 Calculations involving acids and bases 18.3 pH curves 6.1 Collision theory and rates of reaction 16.1 Rate expression and reaction mechanism 16.2 Activation energy 6.5 hours 19.1 Electrochemical cells 10.1 Fundamentals of organic chemistry 10.2 Functional group chemistry 20.1 Types of organic reactions 20.2 Synthetic routes 20.3 Stereoisomerism D.1 Pharmaceutical products and drug action D.2 Aspirin and penicillin D.3 Opiates D.4 pH regulation of the stomach D.5 Anti-viral medications D.6 Environmental impact of some medications D.7 Taxol- a chiral auxiliary case study D.8 Nuclear medicine D.9 Drug detection and analysis 6 hours 11 hours Topic 19: Redox processes Topic 10: Organic chemistry Topic 20: Organic chemistry Topic 21: Measurement and analysis Option D: Medicinal Chemistry The group 4 project 90 In one week there 2-3 are 5.1 Measuring energy changes 5.2 Hess’s law 5.3 Bond enthalpies 15.1 Energy cycles 15.2 Entropy and spontaneity Topic 6: Chemical kinetics Topic 16: Chemical kinetics 2. Allocated time 7 hours 10 hours 7 hours 6 hours 12 hours 2 hours 25 hours minutes. classes. Assessment instruments to be used Resources List the main resources to be used, including information technology if applicable. The same assessments as The science department recently those in year 1 will be used acquired a classroom set of TIin year 2, however more Inspire devices that will soon be frequent emphasis will be accompanied by probes for placed on preparation for chemistry and physical science the upcoming external classes. assessment, as well. As the IB guides say, “The group 4 project is a collaborative activity where students from different group 4 subjects work together on a scientific or technological topic, allowing for concepts and perceptions from across the disciplines to be shared in line with aim 10—that is, to ‘encourage an understanding of the relationships between scientific disciplines and the overarching nature of the scientific method.’” Describe how you will organize this activity. Indicate the timeline and subjects involved, if applicable. The Westlake High School group 4 project will be a collaboration between the IB Biology, IB Physics, and IB Chemistry courses. Our school has acquired solar panels that have not been used. We would like to use them to construct a solar-powered greenhouse with built-in water filtration and collection system. While all group 4 students will participate in different aspects of the project, IB chemistry students will research and teach other students about the solar panels and the chemistry behind the panels. The IB chemistry students will also lead the water filtration/purification process using what they know about separation of mixtures and possible contaminants in the ground/rain water. Students in the chemistry course will be responsible for consulting in the decision of fertilizers, waste materials, and water testing. All students will engage in the engineering of the project (possibly with mathematics students). The project will eventually produce herbs, flowers, and possible vegetables that can be marketed in the community or donated to local food banks. The initiation/designing process should be completed by May of 2017, while the building and implementation on school grounds should be completed by May of 2018, when students should maintain, but another group 4 project or extension of this project should begin. Approval will be secured through the Fulton County Facilities office and Funding will be applied for from the Atlantabased Captain Planet Foundation, which offers grants for garden/greenhouse-type projects in the K-12 Atlanta area. More information can be found at http://captainplanetfoundation.org/apply-for-grants/. Students will also have access to local universities that Westlake currently has working relationships, like Georgia Tech and Georgia State University. The Atlanta Science Festival is also an option to help connect the students with local professional scientists in a field that would help them with the project. More information can be found at http://atlantasciencefestival.org/. Aim 7 will be addressed while students research solar panels and reach out to the local universities for help, if needed. Students also may be asked to communicate the project to the community. Aim 8 will be addressed when the students test rain and groundwater for contaminants and discuss where these contaminants came from, whether it be the school or local industrial plants. Also, students will have to understand the importance of renewable energy and food sources throughout the globe. The project could eventually extend into projects that could help impoverished areas with food production or water purification using renewable energy sources. Aim 10 will be addressed when the students work with other science disciplines and other aspects of the IB program. They will see how the project cannot be completed without multiple subjects and expertise working together for a common goal. In total, at least 10 hours of teaching time will be devoted to this project for each cohort of students. Outside work and other practical activities may be aligned with the project, to allow more time exploring the project. For example, if testing the water samples can be used as a practical activity, the data could be utilized in the group 4 project. 3. IB practical work and the internal assessment requirement to be completed during the course As you know, students should undergo 40 hours (at standard level) or 60 hours (at higher level) of practical work related to the syllabus. Use the table below to indicate the name of the experiment you would propose for the different topics in the syllabus. Indicate which experiments you would use for assessing each of the internal assessment criteria—design (D), data collection and processing (DCP) and conclusion and evaluation (CE). An example is given. Add as many rows as necessary. Name of the topic Experiment Indicate the experiments you would use for assessing design (D), data collection and processing (DCP), and conclusion and evaluation (CE) (use D, DCP or CE) Acids and bases Titration Topic 11: Measurement and data processing Percent by mass: amount of sugar in chewing gum D Yes Topic 11: Measurement and data processing Visible spectroscopy (Beer’s law) DCP Yes Topic 11: Measurement and data processing Greenhouse gases database/spectroscopic analysis DCP Yes Topic 1: Stoichiometric relationships Formula of a hydrate CE No Topic 1: Stoichiometric relationships Formation of magnesium oxide n/a No Topic 1: Stoichiometric relationships Determination of the gas constant, R D No Topic 9: Redox processes Gravimetric determination of copper product from CE reaction between copper (II) nitrate and aluminum Yes Topic 9: Redox processes Redox titration virtual lab n/a Yes Topic 9: Redox processes Activity series wet lab with virtual lab pre-lab assignment CE Yes Topic 9: Redox processes Engineering challenge: creation of simple battery using household materials D, DCP, CE Yes Black box challenge with gold foil simulation D, DCP, CE Yes Cloud chambers with alpha-emitting particles n/a Yes Topic 2: Atomic structure Topic 2: Atomic structure DCP Any ICT used? Remember you must use all five within your programme. Yes 40 Application for authorization: Diploma Programme Name of the topic Experiment Indicate the experiments you would use for assessing design (D), data collection and processing (DCP), and conclusion and evaluation (CE) (use D, DCP or CE) Any ICT used? Remember you must use all five within your programme. Topic 2: Atomic structure Flame test DCP No Topic 12: Atomic structure Photoelectron spectroscopy data investigation CE Yes Topic 12: Atomic structure Ionization energy data investigation CE Yes Topic 3: Periodicity Qualitative analysis of elements and trends (wet lab) DCP, CE No Topic 3: Periodicity Periodic trends data investigation CE Yes Topic 13: The periodic table- the transition metals Magnetic salts, a study of transition metals D, DCP, CE No Topic 13: The periodic table- the transition metals Complex ions spectroscopy investigation DCP Yes Topic 4: Chemical bonding and structure Separation of a mixture based on chemical and physical properties D No Topic 4: Chemical bonding and structure Paper chromatography CE No Topic 4: Chemical bonding and structure Simulation: crystal lattices, VSEPR structures, and n/a metallic bonding Yes Topic 14: Chemical bonding and structure Oxygen- paramagnetic or diamagnetic? CE Yes Topic 14: Chemical bonding and structure Simulation and data collection: hybridization DCP Yes Topic 5: Energetics/thermochemistry Calorimetry D, DCP Yes Topic 5: Energetics/thermochemistry Engineering challenge: making the best cold packs D, DCP, CE Yes Topic 5: Energetics/thermochemistry Hess’s Law n/a No Topic 15: Energetics/thermochemistry Calculating enthalpy of crystallization of water D, DCP, CE Yes Topic 7: Equilibrium Engineering challenge: equilibrium rainbow D, DCP, CE Yes 41 Application for authorization: Diploma Programme Name of the topic Experiment Indicate the experiments you would use for assessing design (D), data collection and processing (DCP), and conclusion and evaluation (CE) (use D, DCP or CE) Any ICT used? Remember you must use all five within your programme. Topic 17: Equilibrium Determining the equilibrium constant for an esterification reaction D, CE Yes Topic 8: Acids and bases Titration n/a No Topic 8: Acids and bases Acid rain investigation (includes determination of strong vs weak acids) D, DCP, CE Yes Topic 8: Acids and bases Option D: Medicinal chemistry Effectiveness of antacids DCP, CE No Topic 18: Acids and bases Development of a pH curve (includes comparing pH curve to database) and determination of equivalence point DCP, CE Yes Topic 18: Acids and bases Household buffers investigation CE Yes Topic 6: Chemical kinetics Iodine clock challenge D No Topic 6: Chemical kinetics Effect of a catalyst CE No Topic 16: Chemical kinetics Determination of the order of a reaction DCP, CE Yes Topic 19: Redox processes Hydrolysis of water and using sodium polyacrylate in diapers to show movement of copper ions in D, DCP, CE electrolysis Yes Topic 19: Redox processes Electroplating virtual lab CE Yes Topic 10: Organic chemistry Distillation D, DCP Yes Topic 10: Organic chemistry Making soap with essential oils DCP No Topic 10: Organic chemistry Purification of a solid product CE No Topic 20: Organic chemistry Option D: Medicinal chemistry Extraction of caffeine from coffee D, DCP, CE Yes Topic 20: Organic chemistry Synthesis and characterization of an enantiomer D, DCP, CE Yes 42 Application for authorization: Diploma Programme Name of the topic 4. Experiment Indicate the experiments you would use for assessing design (D), data collection and processing (DCP), and conclusion and evaluation (CE) (use D, DCP or CE) Any ICT used? Remember you must use all five within your programme. Option D: Medicinal chemistry Topic 21: Measurement and analysis Analysis of a medicine using spectral databases D, DCP, CE Yes Option D: Medicinal chemistry Topic 20: Organic chemistry Synthesis of a widely-used drug or medicine D, DCP, CE Yes Laboratory facilities Describe the laboratory and indicate whether it is presently equipped to facilitate the practical work that you have indicated in the chart above. If it is not, indicate the timeline to achieve this objective and describe the safety measures that are applicable. The laboratory is in the classroom, with the classroom desks in the middle of the room. The lab benches (7 total) are surrounding the center of the room. Each lab station has a cabinet to store materials and glassware and many drawers to differentiate classes or groups. Each lab station has 2 gas lines and a sink in the middle, with 2 faucets. In the back of the classroom, there are more cabinets and drawers for extra storage, as well as a larger sink. The back of the room also houses the fume hood (currently not working, but work should be completed by the start of the 2015-16 school year) and the storage room. Before the storage room is a safety shower and eye wash station. The storage room and shower/eye wash station is shared with the adjacent classroom. The laboratory is equipped with two fire extinguishers, a fire blanket, a gas and water shut-off valve, and a goggle sanitation cabinet. Most of the equipment needed for the practical work is already present, except burets for titrations, an oven for drying samples, and a vacuum filtration system. Burets will likely be purchased in the next school year, but an oven and vacuum filtration system will have to be discussed as part of the IB implementation process. 43 Application for authorization: Diploma Programme 5. Other resources Indicate what other resources the school has to support the implementation of the subject and what plans there are to improve them, if needed. Westlake High School has laboratory classrooms with demo tables and lab stations, exceptional access to technology in magnet classrooms, and a supportive school board and community should more resources be necessary. 6. Links to TOK You are expected to explore links between the topics of your subject and TOK. As an example of how you would do this, choose one topic from your course outline that would allow your students to make links with TOK. Describe how you would plan the lesson. Topic Topic 10/20: Organic Chemistry 20.2 Synthetic Routes Link with TOK (including description of lesson plan) When students begin to explore the aspects of the synthesis of organic compounds, I would like to structure one or two lessons into a debate. This will allow students to research both the benefits and down-sides of laboratory synthesis of compounds. Students would be instructed to stay close to the content, and content explanation would play a role in the debate itself. However, students would also be asked to develop arguments for and against the synthesis and manufacture of organic compounds, with links to historical evidence, medicinal evidence, environmental evidence, and political evidence. Students would have to attempt to see this argument from both sides to make adequate points. At the conclusion of the lesson, each student will be asked to write an essay weighing the points made in the class debate and relate the ways of thinking to the real world, discussing how the debate topics translate to real cultural and political issues. Students would use in-class iPads as a resource for research as well as means to present information in the debate. 7. International mindedness Every IB course should contribute to the development of international mindedness in students. As an example of how you would do this, choose one topic from your outline that would allow your students to analyze it from different cultural perspectives. Briefly explain the reason for your choice and what resources you will use to achieve this goal. Topic Topic 2: Atomic structure 2.1 The nuclear atom Contribution to the development of international mindedness (including resources you will use) As a part of the study of nuclear chemistry, students will research the Manhattan Project and discuss how that and other past investigations in science led to the bombing of Hiroshima and Nagasaki at the end of WWII. Students would be asked to format timelines of events and scientific work that led to the atomic bomb. They would then be asked to research the effects of the bombings on the areas in Japan, and investigate the scientific discoveries that led to the atomic bomb from different cultures, including the U.S. and Japan. Other perspectives may be pulled in for further comparison (European countries, Russia, and Middle Eastern countries). Students may be asked to research more current uses of nuclear chemistry for warfare and take a look at the future of this aspect of science in the world. 44 Application for authorization: Diploma Programme 8. Development of the IB learner profile Through the course it is also expected that students will develop the attributes of the IB learner profile. As an example of how you would do this, choose one topic from your course outline and explain how the contents and related skills would pursue the development of any attribute(s) of the IB learner profile that you will identify. Topic Topic 7: Equilibrium 7.1 Equilibrium Contribution to the development of the attribute(s) of the IB learner profile While this course will attempt to develop the IB learner profile with each topic, equilibrium is a topic in which students will have to develop more their open-mindedness. In general, chemical changes are thought to be one-way streets. Students have to be open to a new or different way of thinking to accept that most chemical changes are actually two-way streets that can be manipulated with external methods. 45 Application for authorization: Diploma Programme 46 Application for authorization: Diploma Programme







