Online Electromagnetic Spectrum Activity
advertisement
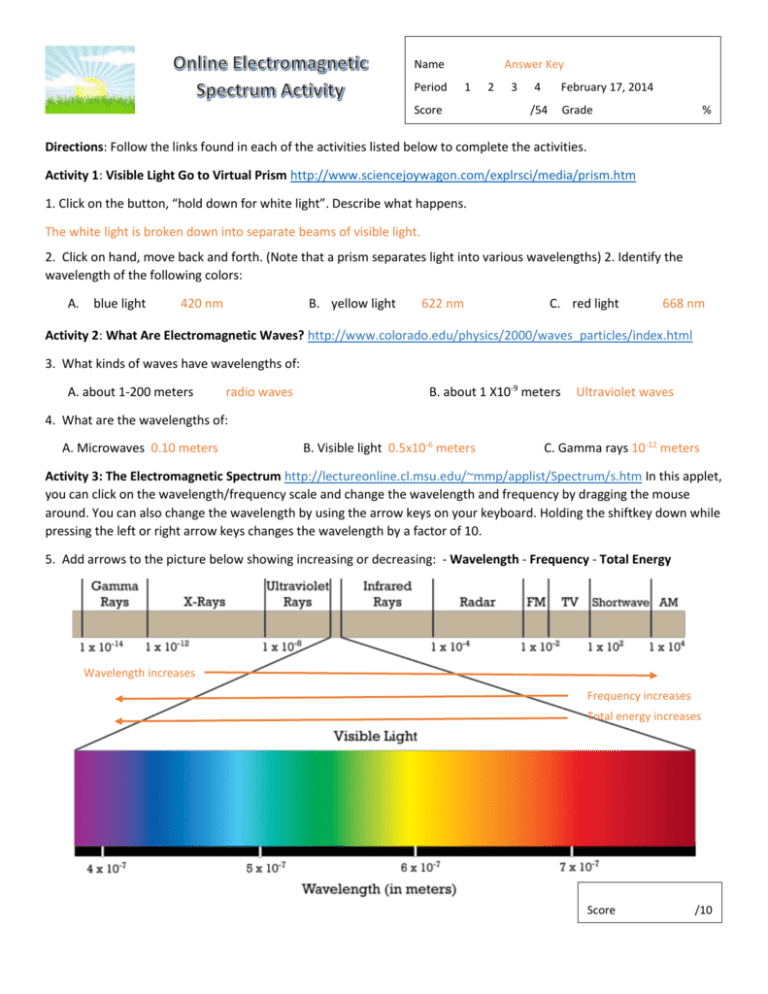
Name Period Answer Key 1 Score 2 3 4 February 17, 2014 /54 Grade % Directions: Follow the links found in each of the activities listed below to complete the activities. Activity 1: Visible Light Go to Virtual Prism http://www.sciencejoywagon.com/explrsci/media/prism.htm 1. Click on the button, “hold down for white light”. Describe what happens. The white light is broken down into separate beams of visible light. 2. Click on hand, move back and forth. (Note that a prism separates light into various wavelengths) 2. Identify the wavelength of the following colors: A. blue light 420 nm B. yellow light 622 nm C. red light 668 nm Activity 2: What Are Electromagnetic Waves? http://www.colorado.edu/physics/2000/waves_particles/index.html 3. What kinds of waves have wavelengths of: A. about 1-200 meters radio waves B. about 1 X10-9 meters Ultraviolet waves 4. What are the wavelengths of: A. Microwaves 0.10 meters B. Visible light 0.5x10-6 meters C. Gamma rays 10-12 meters Activity 3: The Electromagnetic Spectrum http://lectureonline.cl.msu.edu/~mmp/applist/Spectrum/s.htm In this applet, you can click on the wavelength/frequency scale and change the wavelength and frequency by dragging the mouse around. You can also change the wavelength by using the arrow keys on your keyboard. Holding the shiftkey down while pressing the left or right arrow keys changes the wavelength by a factor of 10. 5. Add arrows to the picture below showing increasing or decreasing: - Wavelength - Frequency - Total Energy Wavelength increases Frequency increases Total energy increases Score /10 Name Period 6. What is the relationship between wavelength and frequency? Answer Key 1 2 3 Score 4 February 17, 2014 /54 Grade % The greater the frequency, the smaller the wavelength (they are opposite each other) 7. What type of waves have a wavelength of about a mile? Radio waves 8. What type of electromagnetic radiation would be considered “safe” to be around, absorb? 9. Which color has: the highest frequency? Violet lowest frequency? Visible light Red Activity 4: What is Spectroscopy http://coolcosmos.ipac.caltech.edu/cosmic_classroom/ir_tutorial/spec.html 10. What is the difference between emission spectra and absorption spectra? Emission spectra occurs when an electron drops down to a lower orbit and looses energy; absorption spectra occurs when an electron rises to a higher orbit and absorbs energy. 11. Look at the emission spectrum for the sun and the emission spectrum of hydrogen-20. What do you notice about the two? The emission spectrum for the sun is much longer and has more lines than that of hydrogen-20. 12. Label the 7 colors of the rainbow on the light spectrum below from looking at the webpage. 13. When astronomers find new planets Red outside our solar system they can sometimes tell if there is an atmosphere Orange Yellow and the composition of the atmosphere. How do you think the astronomers can do this? Use this link for help: Green http://www.colorado.edu/physics/2000/ quantumzone/index.html Blue Indigo Each type of gas found in an atmosphere will give off a different emission spectrum. Therefore, by examining these spectrums, Violet astronomers can see what types of gases are found in each atmosphere. 14. From your answers to the questions above, name the relationship between wavelength and frequency in waves that travel at the same velocity like the waves measured in these activities. Wavelength will determine the frequency of the wave. Those with a longer wavelength will have a lower frequency; those with a shorter wavelength will have a higher frequency. Score /16 Name Period How is it Generated 1 Score 15. Complete the table below. Type of Radiation Answer Key Frequency Wavelength <3x109 Hz 1 meter or bigger 2 3 4 /54 February 17, 2014 Grade % Uses Radio Waves Radio waves are also emitted by stars and gases in space. Microwaves Galaxies 3x109 – 3x1012 0.01 – 1 Infrared Waves Solar radiation 3x1012 – 4.3x1014 0.00001 (or 10-6) – 0.01 Light (visible) Waves Solar radiation 4.3x1014 – 7.5x1014 8x10-7 – 4x10-8 Colors Lighting Ultraviolet Waves Solar radiation 7.5x1014 – 3x1017 3x10-7 – 10-8 UV light X-Rays Gases in the Universe 3x1017 – 3x1019 10-8 – 10-12 Gamma Rays The Universe >3x1019 10-12 and smaller AM radio Amateur radio Aircraft communication Microwave ovens TV remote control Night vision goggles Airport security scanners Medical x-rays PET scan Terrestrial gamma-ray flashes Score /28







