TEM CHARACTERIZATION OF PLLA

TEM CHARACTERIZATION OF PLLA-PEO BLOCK COPOLYMERS/
HYDROXYAPATITE NANOCOMPOSITES FOR BIOMEDICAL APLICATIONS
L.M.D. Loiola 1 , L.C.E. da Silva 1 , M.C. Gonçalves 1 , M.I. Felisberti 1
1 Institute of Chemistry, University of Campinas-UNICAMP, Campinas, Brazil
INTRODUCTION
Biomaterials science is a highly interdisciplinary field of research that combines mainly biology and materials science. One of the most important aspects of biomaterials science is characterization, due to the fact that small changes in surface or composition of these materials can modify its interaction with biological systems. Nevertheless these materials, especially polymeric biomaterials, are extremely delicate and therefore difficult to characterize.
The variety of non cytotoxic materials that may be used at this research field is limited.
Poly(L-lactic acid) (PLLA), poly(ethylene oxide) (PEO) and nanohydroxyapatite (nHA) are the most widely studied biomaterials due to their excellent mechanical properties and good cytocompatibility (1). In this work, the triblock copolymer PLLA-PEO-PLLA was reinforced as the polymeric matrix of nHA composites. In order to enhance the copolymer/nHA dispersion, the particles were covered with PEO homopolymer (2). These nanocomposites combine the bioabsorption ability of PLLA, the hydrophilicity of PEO and the capacity of nHA to improve mechanical properties and biocompatibility (3) when applied to a polymeric system.
METHODS
Nanoparticle capping nHA was dispersed in DMF and PEO (Fluka, 8 g mol -1 ) was added. The suspension was stirred for 48h, centrifuged, washed with ethanol and dried under vacuum. According to the thermogravimetric analysis (TGA), nHA was capped with approximately 7 wt % of PEO.
Nanocomposites preparation
PLLA-PEO-PLLA copolymer with a number-average molar mass of 111,000 g mol -1 and
PLLA/PEO block weight proportion of 74/26 was synthesized as described elsewhere (4).
PLLA-PEO-PLLA copolymer was dissolved in benzene (15 w/V %) and 10 wt% of PEOcapped or not capped nHA was added. The suspensions were lyophilized for 48h followed by melting and injection.
TEM Characterization
Nanocomposites were cut in ultrathin sections of approximately 35 nm thick at 100°C using a diamond knife, in a Leica EM FC6 cryo-ultramicrotome and investigated in a Carl
Zeiss Libra 120 transmission electron microscope (120 kV) equipped with an in-column
OMEGA energy filter spectrometer and an Olympus CCD camera 14 bits with 1376 x 1032 resolution.
RESULTS AND DISCUSSION
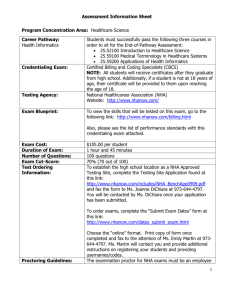
Hydroxyapatite nanoparticles present spherical morphology with a mean particle diameter of 78 ± 39 when not capped and 79 ± 37 nm after capping. Capping has been proven effective through TGA and bright field TEM images (data not shown), however particles are agglomerated even without capping. The diffraction patterns showed that these nanoparticles have a monoclinic crystal structure characteristic of hydroxyapatite and octacalcium phosphate. Therefore, these nanoparticles are a mixture of hydroxiapatite and octacalcium phosphate.
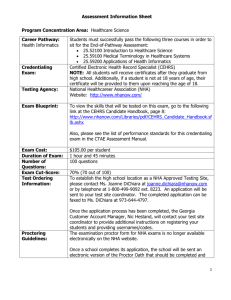
Figure 1 shows that when uncapped nHA is added to the polymer there is no evidence of polymer matrix phase separation. The nHA nanoparticles are spherical and their aggregates are well dispersed in the polymeric matrix. In contrast, when PEO-capped nHA is added to the copolymer, it is possible to observe a large amount of dark domains at the polymeric matrix, while the spherical nHA nanoparticles and their aggregates present non uniform or absent capping. One possible explanation is that the PEO capping is leached out of the nanoparticle surface and is dispersed throughout the polymeric matrix during processing. This is confirmed by the calcium map presented in Figure 2, where it is shown that these dark spots do not contain calcium.
CONCLUSION
TEM has been fundamental to determine the mechanism of particle-polymer compatibilization, through the PEO block, as well as to fully characterize nanoparticles and capping.
ACKNOWLEDGEMENTS
The authors would like to thank FAPESP and INOMAT (CNPq) for funding.
REFERENCES
(1) Kutikov A.B., Acta Biomaterialia 9 (2013) 8354.
(2) Fu SZ, J Biomed Mater Res Part B: Appl Biomater 97B (2011) 74
(3) McMahon RE, J Biomed Mater Res Part B: Appl Biomater 101B (2013) 387
(4) Trinca RB, J Appl Polym Sci 131 (2014) 40419
Figure 1- Bright field image of the nanocomposites containing 10 wt% of not capped
(left) and PEO-capped (right) hydroxyapatite. Inserts: electron diffraction patterns.
Figure 2
– Bright field image (left) and calcium map (right) of the PEO-capped hydroxyapatite nanocomposite. Insert: electron energy loss spectra.