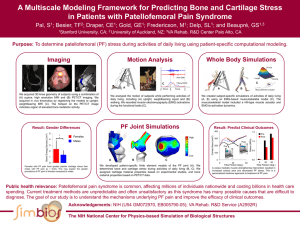
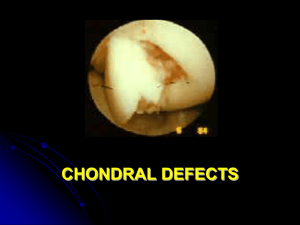
Soluble Mediators in Cartilage Regeneration
advertisement

Soluble Mediators in Cartilage Regeneration Jan Willem van Rijswijk Department of Orthopaedics University Medical Center Utrecht December 2012 Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Utrecht University by Jan Willem van Rijswijk, BSc 3157954 Supervisor: Anika Tsuchida, MD Department of Orthopaedics, University Medical Center Utrecht Examiner: Laura Creemers, PhD Department of Orthopaedics, University Medical Center Utrecht 2 Contents Acronyms ................................................................................................................................................................ 3 Introduction ............................................................................................................................................................ 4 Articular cartilage biology ....................................................................................................................................... 4 Natural history and Epidemiology ........................................................................................................................... 6 Treatment ............................................................................................................................................................... 7 Joint homeostasis .................................................................................................................................................... 8 Soluble mediators in cartilage degeneration .......................................................................................................... 9 The role of soluble mediators in cartilage regeneration ....................................................................................... 12 Summary and Discussion ...................................................................................................................................... 17 References ............................................................................................................................................................ 22 Acronyms ACI ADAM-TS BMP CPC DAMPS ECM FGF FGFR ICRS IGF-1 Ihh IL-1 MAC MACI miRNA MMP MSC NFκB OA OPG PTHrP RANK RANKL Sox9 TGFβ TIMPs TLR TNFα Autologous chondrocyte implantation A disintegrin and metalloproteinase with thrombospondin motifs Bone morphogenetic protein Chondrocyte progenitor cell Damage-associated molecular patterns Extracellular matrix Fibroblast growth factor FGF receptor Cartilage Repair Society Insulin-like growth factor-1 Indian hedgehog Interleukin-1 Membrane attack complex Matrix-induced ACI microRNA Matrix metalloproteinase Mesenchymal stem cell Nuclear factor kappa B Osteoarthritis Osteoprotegerin Parathyroid hormone-related protein Receptor activator of NFκB RANK ligand SRY (sex determining region Y)-box 9 Transforming growth factor-beta Tissue inhibitors of MMP Toll-like receptor Tumor necrosis factor-alpha 3 Introduction Articulating joints rely on a complex design to provide smooth and pain free articulation. The sliding surface of bones is covered by a highly organized avascular tissue called articular cartilage. This hyaline cartilage consists mainly of a matrix of type II collagen and proteoglycans and is maintained by sparsely distributed chondrocytes. The chondrocyte is very sensitive and responsive to stressful stimuli. Joint trauma leads to a disturbance in the highly regulated joint homeostasis, activation of chondrocytes, and a phenotypic shift causing cartilage destruction. Cartilage has a limited intrinsic capacity for repair and untreated, initially focal, lesions may eventually lead to development of osteoarthritis (OA) in which the whole joint, encompassing the cartilage, synovium, and underlying bone, is either involved or affected. Severe pain, loss of movement, and a limited intrinsic regenerative potential of cartilage after degradation currently make OA one of the most important challenges in orthopedic surgery. Existing repair strategies result in cartilage of inferior quality as compared to native cartilage. Understanding cartilage regeneration is essential for improved repair of articular cartilage defects. The present thesis will summarize the changes in intra-articular homeostasis after focal defects of cartilage and osteoarthritis, and focus on the role of soluble mediators in cartilage regeneration. Articular cartilage biology In order to fully appreciate the challenges in understanding cartilage disease progress and improving its regeneration, this first section will cover the normal function and biology of articular cartilage. Healthy articular cartilage is required for the joint’s basic function: articulation. Pain-free, smooth articulation is facilitated by a thin layer of smooth, near frictionless, hyaline cartilage at the articulating ends of bones [1]. Moreover, friction is even further decreased in combination with the lubricating properties of synovial fluid [2]. Adult articular cartilage is an avascular and aneural tissue that consists of an extracellular matrix (ECM) maintained by a relatively low number of isolated chondrocytes. The ECM mainly consists of two components: collagen type II and glycosaminoglycans, which account for 60 and 30% of the dry weight of adult human cartilage, respectively. Water however, comprises up to 80% of the wet weight [1]. When cut longitudinally, four histologically distinct layers can be recognized in cartilage, from the articulating surface towards the subchondral bone [3]. The top layer is called the superficial zone and consists of several layers of cells with a flattened disc-like appearance, including chondrocytes and progenitor cells [4]. The ECM in the superficial zone contains randomly organized horizontal bundles of thick collagen fibers, parallel to the plane of the articular surface, and little proteoglycans. This layer is thought to deal with horizontal shear forces [5]. In the middle zone, few increasingly rounded chondrocytes are found within a matrix consisting of proteoglycans and collagen. The collagen fibers form a transitional network between the horizontal superficial layer and the vertical deep layer collagen fiber orientation. The increase in proteoglycans attracts high amounts of water which together with the collagen act as a mechanical cushion to absorb impact energy. The stiffer deep zone is characterized by high proteoglycan content, vertically aligned collagen fibers, and clusters of chondrocytes (chondrons) orientated along the vertical axis [3]. The bottom calcified layer of adult articular cartilage is separated from the upper layers by the tidemark and provides a biomechanical junction into the underlying 4 subchondral bone, which on a microscopic level shows direct coupling through collagen fibers [5]. These matrix molecules combined with a highly organized architecture are essential for the mechanical properties of articular cartilage, which requires a smooth, low friction surface, and stiffness against compression, forming a cushion to protect the underlying bone from high mechanical peak stress [1]. The cartilage matrix is maintained by chondrocytes and possibly a progenitor cell population which take up only about 5% of the cartilage volume [4, 5]. Chondrocytes are very sensitive to changes in the ECM, presence of soluble mediators in the matrix, but also changes in mechanical loading. In response to these changes, matrix components can be either synthesized or degraded. Matrix turnover is a slow process regulated by catabolic and anabolic cytokines including interleukin-1 (IL-1) and insulin-like growth factor-1 (IGF-1), respectively [1]. These cytokines in turn can mediate ECM remodeling by inducing expression of collagen and proteoglycans, or matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), and the aggrecanases -4 and -5 [5]. In healthy cartilage, synthesis and degradation are in a delicate balance and the slightest disturbance can cause serious damage [1, 5]. Because articular cartilage lacks vascularization and innervation it depends on oxygenation and nutrition by diffusion of synovial fluid and by the underlying subchondral bone. Synovium The synovial tissue is the blood vessel-rich inner membrane that lines the joint cavity. The intimal layer facing the articular cartilage consists of one to three cell layers of type A and type B synovial intimal cells. The type A synoviocytes are tissue macrophages with antigen presenting capabilities, actively clearing cell debris and matrix degradation products in the joint cavity [6]. Type B cells are fibroblast-like and involved in production of hyaluronic acid, collagen and fibronectin for use in both the loose connective tissue and synovial fluid [7]. This layer is thought to play an important role in joint homeostasis by providing nutrition to articular chondrocytes, clearance of intra-articular debris, production of lubricating synovial fluid, and regulation of immune function [6-8]. Subchondral bone Subchondral bone consists of the bone plate underlying the cartilage and is supported by a network of trabecular bone. Subchondral bone and cartilage are biomechanically linked and the mechanical properties of cartilage are probably dependent on the quality of the subchondral bone and the trabecular network, and vice versa. Healthy cartilage requires a gradual stiffness gradient for optimal distribution and damping of mechanical loading [9, 10]. In addition to mechanical load transfer, this region is also involved in cartilage nutrition through structures called chondro-osseous junctions and/or micropores. These junctions are prolongations of deep layer cartilage that extend through the calcified cartilage layer and contact the underlying bone or marrow spaces. This contact implies a potential direct molecular communication pathway 5 between bone cells, chondrocytes and synoviocytes. Joint homeostasis may thus be directly influenced by cytokines and growth factors released from subchondral bone [11, 12] Natural history and Epidemiology The beginning of the natural history of OA is largely unknown but several factors may predispose joints to the disease. It is most likely a combination of both endogenous risk factors like age, sex, genetics, and systemic factors on one side, and environmental factors with injury, overload, and joint instability as most common pathways on the other side. All articular joints including, fingers, knees, hips, and the spine can be affected, especially the weight-bearing joints. OA has potentially devastating effects on these joints and on the overall quality of life because of the involvement of pain, restricted mobility, and limitations of daily living activities [13]. OA also represents a large economic burden mainly because of the previously described restrictions, but also coexisting disorders, and the high cost of treatment, and is expected to increase in the future because of the ageing population [14]. Knees, hands, and hips are commonly involved in OA of which the knee is most frequently affected in the general adult population. The Framingham OA study (1983-1985) found radiographic knee OA in 34% of women and 31% of men between the ages of 63 and 93 living in the United States. Radiographic knee OA is defined as the radiological observation of joint space narrowing, multiple osteophyte formation, and subchondral sclerosis. In women the prevalence of OA increased significantly with age, they showed 25% radiographic OA at ages 63-69 and 52,6% at age ≥80, while at the same time in men this increase was far less pronounced, with 30,4% displaying radiographic OA at ages 63-69 and 32,6% at age ≥80. Symptomatic knee OA prevalence, associated with disability, was also more common in women than in men between age 63 and 93 years (11 vs. 7%) [15]. These numbers highlight the high prevalence of OA, and the significant effect of gender. The onset of OA can often be traced back to joint trauma involving mechanical overload. Previous knee injury significantly increases the relative risk of severe radiographic and symptomatic knee OA in both men and women (relative risk for men, 3,46 vs. women, 2,18) [15]. Sport accidents (especially skiing and football) are the main cause of acute traumatic damage in over halve of the cases. Accidents during daily living (work and at home) account for another major fraction of cases [16, 17]. In 10-39% of the patients with cartilage damage however the cause is unknown [16-18]. Daily wear and tear of the joints is also likely to contribute; hence age as risk factor. But also obesity and diabetes are strong risk factors for developing OA. In the Framingham OA study, pre-existing obesity strongly predicted the risk of later severe radiographic (RR men vs. women: 1,86 vs. 3,16) and symptomatic OA in men and women [15]. In addition to the obvious mechanical effects of obesity on articular joints, it may promote a low grade inflammation in joints and production of adipokines, predisposing to OA development [19]. Disorders to ligaments supporting the joint can also cause instability and altered mechanical loads. 6 In most cases, OA is initiated as a focal area of damage located at a load-bearing area of the cartilage and is frequently not isolated to a single joint. The damage at this point is likely limited to disruptions in cartilage matrix in the superficial layers and mostly asymptomatic with some minor temporary swelling, local pain, locking, and catching [20]. The lesion size is dependent of the type and the severity of the trauma, and according to the Cartilage Repair Society (ICRS) there are four anatomical gradations of the depth of cartilage injury [21]. ICRS grade I describes near normal cartilage with only superficial defects, whereas in grade II lesions the defect extends down to up to 50% of the cartilage depth. Grade III is a lesion with full thickness cartilage damage up to but not including the subchondral bone, while the subchondral bone is also penetrated in grade IV lesions. Smaller defects may eventually completely regenerate, whereas defects larger than 3 mm in diameter do not repair spontaneously [20]. In these larger lesions the failing repair processes lead to a cycle of increasing loss of homeostasis and cartilage matrix which may last for years or decades. Over time, the loss of cartilage results in narrowing of the joint space which is visible on radiographs. At this stage, surrounding innervated tissues are involved in causing pain, while loss of smooth cartilage causes the known clinical problems including joint pain, inactivity stiffness, restricted movement, and crepitus [22]. Many patients that have become symptomatic will consult a physician. OA however is not always easy to diagnose, e.g. there is only a weak correlation between radiographic changes in the joint, the gold standard, and symptomatic OA progression, so it’s possible that some patients show no radiographic changes but display all the symptoms, but it is also possible that radiographic OA is diagnosed while the patient is free of symptoms [22]. For a more accurate diagnosis arthroscopy is required. During the procedure the source of symptoms and a prognosis can often be determined [22]. Accurate diagnosis is very important because early detection improves the chances of effective treatment [23]. Treatment When the patient is presented at the clinic and is diagnosed with osteoarthritis, the first treatment is aimed at reduction of pain and stiffness to maintain or restore mobility and improve the quality of life. The other goal of treatment is to prevent the progression of cartilage degradation and to regenerate damaged tissue, when possible. Therapy can be pharmacological for symptom relieve, non-pharmacological, or surgical, but more commonly is a combination, depending on the patients’ state [24]. The first step always consists of informing the patient on OA and on the importance of changes in lifestyle like weight loss in obese and therapeutic exercise to reduce pain and symptoms [24]. Patients may also use simple painkillers and topical agents. More severe cases are also treated pharmacologically which involves the use of anti-inflammatory drugs and other supervised therapies. Although these procedures (temporarily) alleviate joint pain, they do not induce cartilage repair and without further treatment this can result in progressive cartilage destruction. The development of pharmacological therapy targeted at disease causing factors is challenged by the fact that OA is a multifactorial disease, induced by a combination of mechanical and complex biochemical interplay [24]. However, many new targeted pharmacological approaches are in development and 7 have passed pre-clinical stages. The drugs in phase I and II clinical trials include aggrecanase inhibitors, iNOS inhibitors, and recombinant growth factors like FGFs and BMPs [25]. The most commonly used surgical method for treatment of severe symptomatic chondral defects is the microfracture technique. This technique relies, together with the drilling method, on penetration of the subchondral bone plate to access underlying blood vessels and bone marrow. The resulting fibrin clot at the surface of the defect is then repopulated by migrating bone marrow stem cells. However, the fibrocartilage that is formed by these cells is of inferior quality and prone to deterioration [20]. Other surgical treatments include autologous cartilage grafts (mosaicplasty) and chondrocyte implantation (ACI) and have resulted in varied long-term outcomes, in part due to the unpredictable nature of repair. During mosaicplasty, cartilage plugs are excised from ‘non-load bearing’ regions of the articular cartilage and transplanted to the site of the lesion. ACI is an early tissue engineering technique in which chondrocytes are also isolated from a non-load bearing area, but then expanded in vitro over the course of 2 to 3 weeks, and subsequently injected into the defect, kept in place by a sutured autologous periosteal patch [26]. The ACI procedure yields good long-term results in a large percentage of patients, and rapidly evolved into new, more efficient techniques like matrixinduced autologous chondrocyte implantation (MACI). Like ACI, MACI requires initial surgery to isolate autologous chondrocytes; however the cells are now cultured on a three-dimensional bio-scaffold which better preserves the chondrocyte phenotype. After several weeks the scaffold is glued onto the calcified cartilage layer with fibrin gel. MACI has resulted in promising short term results, but long-term outcome is still unknown and further investigation is required [27, 28]. Mosaicplasty, ACI, and MACI use autologous cells that are isolated during arthroscopic examination and this requires the sacrifice of cartilage at a non-load bearing area. It is suggested however that the commonly recommended sites for osteochondral harvest are in fact active sites of contact pressure and thus involved in load bearing [29], and that this may lead to donor site morbidity. Sacrifice of perfectly fine cartilage should thus preferably be avoided and with the increasing number of papers on stem cells in cartilage regeneration and the effects of different anabolic factors this may soon become possible. Stem cells have been found in cartilage and surrounding tissues and display chondrogenic capacity and regenerative potential when stimulated with the appropriate growth factors [4, 30]. Furthermore, it is unclear in what way the cells seeded in the graft contribute to repair or regeneration. A third generation of techniques, using biodegradable scaffolds for in situ tissue engineering might represent the next step. These scaffolds can be seeded with MSCs and/or biological cues to ‘guide’ derived and migrating cells towards chondrogenesis [31]. Joint homeostasis One of the possible reasons why current treatments for cartilage defects fail to deliver is the fact that most therapies focus exclusively on the cartilage even though the entire joint is involved. Cartilage is only one of the players and restoration of this structure alone will not result in restoration of the whole joint function. In healthy joints the interactions between cartilage, the synovium, synovial fluid, subchondral bone, but also the 8 menisci and ligaments, create a highly organized dynamic environment with a balanced metabolism [32]. Cartilage, synovium and subchondral bone are known to directly communicate through the synovial fluid and chondro-osseous junctions. Anabolic and catabolic factors, anti-inflammatory and pro-inflammatory components are all exchanged between these structures and kept in a highly balanced state to ensure that together they provide pain-free and smooth articulation. The concept of joint homeostasis is used to describe this delicate equilibrium, in which joint tissues are capable of responding adequately to changes that fall within physiological limits. Loss of homeostasis is known to precede the onset of OA and is likely to be influenced by extrinsic mechanical and biological factors such as loading, weight, age, and several predisposing disorders. Changes in e.g. mechanical load distribution that lie beyond the normal physiological range will disrupt the local matrix and disturb the metabolism of affected cells [1]. When a damaging factor is sufficiently forceful or repeated for a sufficient duration of time, the metabolic equilibrium will shift towards an inflammatory catabolic state and eventually leads to the alterations in joint homeostasis with involvement of the entire joint. The loss of homeostasis directs the joint into a destructive cycle wherein matrix components are being degraded much faster than they are synthesized, and where cartilage degradation is maintained by synovial inflammation and subchondral bone remodeling [1, 23]. Soluble mediators in cartilage degeneration Focal cartilage defects are often found after impact trauma and predispose to OA development. Mechanical forces disrupt the ECM and cause cell deformation. Pathological loads and stresses that result in matrix disruption, but also loads that produce no visible damage, may be translated to the chondrocyte by integrinmatrix interactions, which are essential for differentiation and viability, demonstrating the importance of the cell environment [33, 34]. Indeed, a decrease in chondrocyte survival is often observed in vitro and in vivo in the early phase after cartilage damage [35-38]. Traumatic impact causes death by necrosis primarily in the superficial cartilage zone and some apoptosis in deeper layers as the lesion progresses [35, 37, 39, 40]. In chondrocytes outside the necrotic and apoptotic zone, the mechanical load is mechano-transduced and may activate or increase the expression of a numerous genes involved in inflammation and cartilage degradation [41]. Within hours of initiation, the expression of cytokines including tumor necrosis factor (TNF)α, interleukin (IL)-1β, IL-6, IL-1 receptor antagonist (IL-1Ra) [42], and proteases including matrix-metalloproteinases (MMP-1, MMP-2, MMP-3, MMP-9, MMP-13) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAM-TS) dominates the chondrocyte expression pattern [39, 41, 43]. The result is a disturbance in the joint homeostasis in which a shift to catabolic processes is observed. Cartilage ECM synthesis is inhibited while matrix degradation is increased by the production of proteases. Localized loss of proteoglycans is first observed and is followed by collagen type II degradation, leading to cartilage softening and further matrix instability [42]. ADAMTS-4 and -5 appear to be responsible for the majority of proteoglycan degrading [44], whereas collagen type II is mainly degraded by MMP-1 and MMP-13 9 [45]. The increased release of matrix fragments from the cartilage surface into the synovial fluid can stimulate the production of inflammatory cytokines and MMPs in the synovial membrane [46] which may last for decades [43]. Synovial membrane inflammation is found in 50-90% of patients with early or late stage OA [8, 47] and is characterized by inflammation with production of pro-inflammatory cytokines and mediators, infiltration of leukocytes, intimal thickening, and fibrosis. Synovitis is now recognized as a key factor in OA pathology wherein cartilage alterations fuel synovitis, which in turn amplifies cartilage destruction. The exact mechanism by which the synovium contributes to cartilage degradation is unknown, but it is thought that the matrix fragments released during cartilage breakdown may act as damage-associated molecular patterns (DAMPS) on Toll-like receptors (TLR) [48]. Synovial fibroblasts, macrophages, but also chondrocytes are known to express these innate immune receptors and upon activation induce the TLR-activated transcription factor NFκB and subsequent production of inflammatory cytokines and MMPs. In the synovium this leads to increased mononuclear cell infiltration and neovascularization. Thus, involvement of the synovial membrane generally intensifies cartilage degradation by increasing the inflammatory response [48]. Impact trauma may also lead to blood or serum leaking into the joint, changing the composition of the synovial fluid and introducing innate immune factors like the complement system. Complement components have indeed been observed in both healthy and diseased cartilage [49, 50]. Synovial fluids from patients with early OA show increased levels of C3a and C5b-9 (MAC), indicating complement activation. The source of increased complement activity may not necessarily be blood, as both cartilage and the synovium are able to produce complement proteins and change their activity in cartilage disease [49, 51]. The trigger of increased activity is thought to come from cartilage ECM degradation products. Collagen binding proteins have been found to be potent activators of the classical and alternative complement pathways, suggesting they may contribute to the disease progression [51, 52]. Complement may contribute by promoting pore-forming membrane attack complex (MAC)-induced cell death or membrane instability in chondrocytes, the latter resulting in the increased expression of degradative enzymes, inflammatory cytokines, and COX-2 [51]. Complement activation also stimulates chemotaxis of leukocytes, which is reflected by synovitis. The importance of complement in the pathogenesis after a cartilage defect is underlined by a study in which C5-deficient mice showed substantially attenuated cartilage degradation and synovitis in an induced OA model as compared to C5-sufficient mice [51]. OA involves the entire joint, and starting from the moment of injury, the mechanical abilities of the cartilage to transport loads to subchondral bone are altered. It is however also proposed that initiating events in the bone lead to changes in cartilage load transmission and subsequent cartilage degradation. In either way, cartilage remodeling and bone remodeling coincide and communication pathways between the tissues have been demonstrated [11, 12]. It is suggested that pathological mechanic loads translate to osteoclasts, as it does to chondrocytes, and induces an imbalance in bone formation and resorption. Initial mechanical stress however does not appear to be a prerequisite to induce these changes [53]. Nonetheless, the shift in bone homeostasis results in high bone resorption in early OA, likely to be mediated by phenotypically altered osteoblasts. These changes in bone remodeling are in part due to increased activity of IGF-1 and TGF-β, which in turn are 10 regulated by cytokines such as IL-1β, IL-6 and TNFα [9, 54]. It is hypothesized that these cytokines, produced by osteoblasts, diffuse through channels in the calcified cartilage into the deep layer of the cartilage, and research has already shown that factors secreted by OA bone cells are able to influence cartilage metabolism [55, 56], and that OA chondrocytes can affect subchondral bone metabolism [57]. Whether one process precedes the other or vice versa remains elusive but understanding this is essential for the development of treatments. Besides IGF-1 and TGF-β, other proteins like receptor activator of NFκB (RANK), RANK ligand (RANKL), and osteoprotegerin (OPG) similarly control subchondral bone remodeling and might also be involved in cartilage metabolism [58, 59]. The ratio at which RANKL and OPG are produced in subchondral bone is impaired during OA and leads to changes in bone remodeling and cartilage homeostasis [59]. Changes in subchondral bone are also mediated by TGF-β/BMP signaling [9, 60]. In line with TGF-β/BMP signaling, a cross-talking pathway represented by Wnt/β-catenin is involved in osteoblast differentiation and bone remodeling. Activation of the Wnt pathway leads to increased levels of stable β-catenin in chondrocytes in vitro, inducing a catabolic shift with increased expression of MMP-3, -13, and ADAMTS-4, and 5 [61]. Increased levels of nuclear β-catenin in OA-like chondrocytes in vivo in a guinea pig model suggest the direct involvement of the Wnt/β-catenin pathway in cartilage remodeling and degradation [61]. These persisting changes in both cartilage and bone homeostasis, in collaboration with the synovium, lead to a vicious cycle of altered biomechanical properties, in which inflammation, cell death, and matrix degeneration leads to the progression of cartilage damage to a ‘point of no return’. A recent study suggests that ‘about half of the patients with traumatic joint injury develop OA’ [62]. The process of OA development after joint injury appears to be a continuous process in which normal joint homeostasis is increasingly lost, catabolic activities dominate over anabolic processes, and intrinsic repair fails. Most inflammatory mediators and effectors involved in the joint response after injury remain present during OA progression [50]. Several temporospatial differences in expression have been observed however. Progression of OA by progressive degradation of cartilage collagen is mediated by MMP-13 expression, which is found in increasing concentrations with increasing stages of OA [63]. Mild synovitis is present in all stages from trauma to late-stage OA [48], but the evolution of synovitis with the progression of OA appears to be variable and may depend on an unknown factor [64]. Several studies report an increase in synovial inflammation as disease progresses, with the greatest intensity during late-stage OA [65], while others observe the opposite, with increased numbers of infiltrating macrophages, lymphocytes, and neovessels in early OA [64, 66]. Nonetheless, increased levels of secreted pro-inflammatory cytokines (primarily IL-1β and TNFα) in the synovial membrane activate other synovial cells and chondrocytes and induce expression of inflammatory mediators including iNOS, COX-2, and the proteases MMP-3, -9, -13, and ADAMTS-4 [67]. Besides the proteolytic properties of the MMPs, they are also involved in regulating the activity of other proteases and growth factors [68]. More recently several microRNAs (miRNAs) have been identified that show differential expression in OA cartilage and bone tissue when compared to healthy joints. Deregulation of miRNAs including miR-9, -98, -146 [69], and miR-455 [70] is thought to contribute to cartilage destruction by altering gene expression and thereby 11 regulating proteins as IL-1β, TNF-α, MMP-13, and TGF-β. It thus appears that miRNAs also play a role in changing cartilage homeostasis and mediating cartilage destruction [69-71]. In summary, the process leading to cartilage degradation and OA is multifactorial, altering expression and secretion of numerous genes, factors and soluble mediators in the whole joint. Mechanical overloading can cause heterogeneous lesions in all layers including the bone plate. Chondrocytes are killed on impact and disruption of the cartilage matrix leads to dysfunction of surrounding cells. Increased catabolic activity, matrix degradation fragments and released pro-inflammatory cytokines spread through the joint. TNF-α and IL-1β appear to be the main mediators of inflammation and cartilage destruction by inducing expression of NFκBregulated inflammatory genes in the cartilage, synovial membrane, and subchondral bone [45]. Increased expression of ADAMTS-4 and -5, and several MMPs, most importantly MMP-13, leads to destruction of cartilage matrix while homeostatic changes in the cartilage decrease matrix synthesis. The release of cytokines and matrix fragments into the synovial fluid enhances or initiates synovitis by complement activation and DAMPS-induced TLR activation, which in turn increase cell death and inflammation intensity by the release of more cytokines and proteases. OA synovial intimal cells are an important driving factor in disease progress. Parallel to, or even preceding the events in the cartilage and synovium is the aberrant remodeling of bone tissue by imbalanced IGF-1, TGF-β/BMP, RANKL, and OPG expression. Remodeling of the bone alters its loadbearing properties which are reflected in mechanical changes in the overlying cartilage. Furthermore, the expressed factors in the subchondral bone are able to directly mediate changes in chondrocyte metabolism through communicating channels. Because of the lack of effective endogenous cartilage repair, cartilage matrix is increasingly degraded, fueling inflammation in the three joint tissues by release of ECM fragments and changes in load transduction. Further understanding of the timing of events in the joint is needed to grasp the complex pathways that are responsible for homeostatic changes and disease in OA. The role of soluble mediators in cartilage regeneration OA causes irreversible loss of cartilage by increasing inflammation and failing attempts to repair. The low intrinsic capacity for self-repair in adult cartilage make it one of the key obstacles in orthopedic treatment. In a joint environment where homeostasis is disrupted and catabolic factors dominate, the only way to halt progression and start regeneration appears to be to restore homeostasis before irreversible damage is inflicted. Unfortunately, this means necessity for early treatment; a period in which most individuals are asymptomatic and do not present themselves for treatment. Treatment during this period of relatively normal joint homeostasis positively influences the outcome of cartilage formation. In contrast, late treatment of older defects, with highly disturbed homeostasis, does not lead to repair. This indicates the presence of a factor, or a change in ratio of factors in cartilage that affect the regenerative potential [23]. It is well known that while mature articular cartilage has low potential for repair, fetal and immature cartilage have the ability to spontaneously regenerate [72]. In some species, like the eastern newt and the axolotl salamander, adult animals maintain the capacity to completely regenerate damaged cartilage and even 12 complete limb and tail structures [73, 74]. Mechanisms involved in cartilage regeneration in these animals are remnants of embryonic cartilage development. It was found e.g. that the transcription factor SRY-box 9 (Sox9), which is essential for early chondrocyte differentiation, was increased over time after injury and associated with increased matrix synthesis, suggesting reinstatement of latent chondrogenic capacities [73]. In adult mice cartilage, Sox9 appears to have taken on a central role in mediating the expression of numerous chondrocytespecific genes and maintaining a healthy cartilage homeostasis [75], and loss of Sox9 expression in OA cartilage may promote loss of cartilage homeostasis by inducing hypertrophy, cell death and decreasing ECM content [75, 76]. On the other hand, articular cartilage lesions lead to local proliferation and matrix expression in Sox9rich chondrocytes in an OA mouse model. These studies suggest that, like in salamanders, mammalian Sox9 is involved in the induction of chondrogenesis in mature cartilage after injury [76]. The repair processes initiated by these chondrocytes however are insufficient at overcoming the progressive degeneration of cartilage. Interestingly, in an OA rabbit model, implantation of Sox9-transduced bone marrow stem cells enhanced cartilage repair by taking on a stable chondrocyte phenotype [77]. Sox9 may also be involved in regulation of vascularization and subchondral bone growth via suppression of several genes including VEGFA, RUNX2, MMP13, and RANKL [78], suggesting that cartilage and bone homeostasis are also linked via Sox9. This is underlined by an in vitro co-culture study in which OA subchondral osteoblasts decreased Sox9 and subsequently COL2A1 gene expression in chondrocytes [56]. Sox9 activity in turn is highly regulated by soluble upstream mediators including bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), IGF-1, parathyroid hormone-related protein (PTHrP), and Indian hedgehog (Ihh) [76]. BMPs are well known powerful bone and cartilage inducing cytokines of the transforming growth factor-beta (TGF-β) superfamily and may regulate Sox9 via BMP-Smad signaling [79]. BMPs anabolic properties in this way contribute to mature joint homeostasis [80]. More specifically, BMP-2 and -7 and its receptor BMPR-1A are detected in the synovial membrane, in normal cartilage, and at lesion borders, and an important role for BMP-2 and -7 has been predicted in cartilage repair [81]. Furthermore, BMP-2 mRNA was found up regulated after cartilage injury [82] and deletion of the BMPR1A gene results in OA-like cartilage degeneration in mice [80]. BMP-2 addition in an OA mouse model leads to an increase in ECM turnover, indicating a role in remodeling or repair [83]. BMP-7 (also known as OP-1) also plays a key role in cartilage homeostasis and enhancing repair by improving chondrocyte survival and improving the quantity and quality of matrix repair after injury [84, 85]. BMP-7 is able to regulate both anabolic and anticatabolic processes at the level of the ligand, receptor, and downstream targets [86], and stimulates production of collagen II, proteoglycans, IGF-1 and the IGF-1 receptor. Conversely, inhibition of the BMP-7 gene leads to depletion of matrix components from the cartilage ECM [86]. BMP-7 belongs to a subgroup of BMPs that also includes BMP-6. This protein is known to promote chondrocyte differentiation in the growth plate and possibly other early chondrocyte metabolic processes. The role of BMP-6 in mature cartilage is unclear but expression at both mRNA and protein level was observed in healthy and OA cartilage, and in vitro stimulation of OA and healthy chondrocytes with BMP-6 stimulated proteoglycan synthesis [87]. These findings suggest 13 that BMPs play a key role in cartilage maintenance, repair, and homeostasis, and appear to do so in a Sox9 dependent manner. BMP-6 however may also influence cartilage repair in a different way. In a study on chondrocyte progenitor cells (CPCs), isolated from late stage OA cartilage, it was demonstrated that these cells migrate to OA cartilage ex vivo and that BMP-6 can serve as a chemoattractant [30]. In combination with TGFβ3, BMP-6 also positively regulates matrix formation by CPCs by increasing collagen type II expression while at the same time reducing collagen type I and MMP13 mRNA expression. Although Sox9 mRNA expression was unaltered after TGFβ3/BMP-6 stimulation, Runx2 expression was reduced and this explains the changes that enhance the chondrogenic potential of CPCs. This transcription factor is found increased in OA cartilage and promotes disease by inducing MMP13 expression [30]. The previous study brings us to the role of progenitor cells in cartilage regeneration. Bone marrow stem cells and adipose derived stem cells have demonstrated chondrogenic potential and have been studied extensively. Furthermore, bone marrow cells are already indirectly clinically applied with moderate success in the microfracturing and drilling therapy, so called marrow stimulation [88]. The use of stem cells to induce tissue regeneration is highly attractive, especially when the native tissue shows low regenerative capacities. Increasing evidence suggests that cartilage itself also contains a large progenitor cell pool [4, 30, 89-91]. These progenitor cells are thought to be remnants of bone growth during childhood in which cartilage is appositioned from the epiphysial plate to increase bone length [89]. This is supported by the apparent distribution of progenitor cells in cartilage, namely primarily in the superficial [4, 89, 91] and middle cartilage zones [91]. The exact role of the progenitor population in mature cartilage is unclear. Several studies demonstrated an increased frequency of stem cell markers in OA cartilage which suggests the involvement of progenitor cells in the course of OA [89, 90]. One explanation for this may be an increase in migratory mesenchymal cells from the bone marrow through micro-fractures that penetrate the subchondral bone, which have been observed in OA joints [90]. In either way, the observed progenitor cells appear multipotent and show a MSC-like differentiation pattern and have the potential to regenerate structural and biomechanical properties of diseased cartilage [30, 90]. These observations suggest that the intrinsic reserve capacity for regeneration of diseased cartilage is larger than previously thought and may provide a basis for regenerative therapies [91]. The presence of a progenitor cell population in the cartilage (or in the underlying bone marrow) also indicates that the use of exogenous cell transplantation may not be necessary for cartilage regeneration. This is emphasized in the previously mentioned study with TGFβ3/BMP-6 as a chemoattractant for CPCs but also in another recent study. Lee et al. demonstrated in a model that acellular bioscaffolds impregnated with TGFβ3 could induce full regeneration of articular cartilage defects [92]. TGFβ3, not specifically known for its homing abilities, was able to recruit stem cells from surrounding joint tissues in a 3D cell migration system, including from bone marrow and adjacent adipose tissue, and induced these cells to undergo chondrogenesis [93]. Thus 14 stimulation of the latent regenerative potential by augmenting progenitor cell homing and enhancing chondrogenic potential seems feasible. Another member of the TGFβ-family, TGFβ1, which is abundantly expressed in normal articular cartilage, is also able to stimulate chondrogenesis in bone marrow progenitor cells in addition to synovial progenitors. Furthermore, TGFβ1 stimulates ECM production and reduces catabolic processes, including MMP13 expression in chondrocytes [94]. TGFβ1 delivery can enhance cartilage homeostasis and regeneration in rabbits [95], and TGFβ1 deficiency leads to OA-like cartilage [94]. High synovial fluid TGFβ1 levels are observed in OA joints and are associated with osteophyte formation and synovial hyperplasia and fibrosis [96]. TGFβ1 thus appears crucial for cartilage homeostasis but at the same time its spatial expression and receptor sensitivity must be highly regulated witnessing the adverse reactions at the osteochondral border and the synovial membrane. Besides BMPs and TGFs several other growth factors are also able to modulate cartilage repair responses. A number of studies demonstrated improved cartilage repair response after stimulation with FGF-2 (bFGF) in vivo and in vitro [97-100]. FGF-2 is a growth factor known to effectively stimulate bone marrow mesenchymal cells to grow and expand, and it is suggested that FGF-2 is involved in cartilaginous repair by stimulating expansion and chondrogenic potential of CPCs in articulating joints [98-100]. It may do so by a dose dependent increase of Sox9 expression in resident cartilage CPCs and their derived chondrocytes [99]. Interestingly, other research has defined FGF-2 as a degenerative mediator in OA, with effects ranging from inhibition of proteoglycan expression to upregulation of Runx2 and MMP13 [101]. It is thought that FGF-2 can mediate effects on CPCs and chondrocytes via different receptors. Binding to the FGF receptor 1 (FGFR1) results in mainly catabolic effects while activation of FGFR3 induces anabolic effects like chondrogenic differentiation and ECM formation. FGF-18, another member in the family, possibly exerts the same effects by activating FGFR3 [102]. FGF2 is secreted by chondrocytes and partly stored in the pericellular matrix. Possibly upon traumatic impact with matrix disruption these factors are released in increased numbers and mediate changes in joint homeostasis [103]. Increased levels of FGF-2 have been detected in OA synovial fluid which decreased when cartilage regeneration was observed [104]. On the other hand, FGF2 deficient mice developed accelerated OA but could be rescued by delivery of exogenous FGF2 [105]. There appears to be a dynamic balance between the levels of FGF-2 and the availability of its receptors FGFR1 and FGFR3. A traumatic mechanical event (and possibly aging) may cause disturbance in this homeostasis with resulting upregulation of catabolic factors [106]. Most of the growth factors and proteins discussed in this chapter mediate their anabolic effects through the Sox9 transcription factor. This is no different in a protein that is often associated with its positive effects on chondrocytes in vivo and in vitro: IGF-1. IGF-1 is secreted locally by chondrocytes, stimulates matrix production in chondrocytes and bone marrow stem cells, and aids in cell survival in part by stimulating Sox9 expression [107, 108]. IGF-1 is also capable of counteracting catabolic effects, e.g. IL-1 and TNFα induced matrix degradation [109, 110]. However, it has been demonstrated that IGF-1 production decreases with age in humans [111], IGF-1 responsiveness also showed an age-related decline in non-human primate cartilage, but 15 had no effect on cell viability [112]. IGF-1 deficiency causes OA-like lesions in cartilage of rats indicating that IGF is required for normal articular cartilage integrity [113]. Furthermore a decrease in IGF-1 response was detected with increasing OA in monkeys [112]. When added exogenously in an OA horse model, IGF-1 facilitated repair of cartilage defects with increased collagen type II production in the deep layers and protection against matrix degradation [109]. A combined growth factor treatment with IGF-1 and BMP-7 in in vitro 3D culture showed even more matrix production than was observed for each factor alone [85]. It is suggested that growth factors like IGF-1, FGF-2, and TGFβ1 are able to modulate each other’s response [114]. Other proteins that target Sox9 and thereby may affect cartilage homeostasis are PTHrP and Ihh. These factors are involved in chondrocyte maturation and endochondral ossificiation [115], and participate in the maintenance of cartilage homeostasis in growing and adult mice in a load-induced fashion [116]. Ihh and PTHrP form a feedback loop to control chondrocyte differentiation and it is suggested that dysregulation of this system may contribute to OA in the form of disturbed hypertrophic chondrocytes [116]. In summary, mature cartilage may possess more intrinsic repair capacities than originally suspected. Many of the growth factors and other proteins involved in chondrogenesis are still expressed in mature articular cartilage and take on regulatory roles. BMP-2, -6, and -7, TGFβ1 and -β3, FGF-2 and -18, and IGF-1 have shown great potential as cartilage anabolic factors involved in cartilage maintenance, repair, and homeostasis. These growth factors not only act on chondrocytes via Sox9 by inducing matrix synthesis, improving matrix quality, and counteracting effect of numerous catabolic mediators, but in addition have similar effects on stem cells. Stimulation of bone marrow and adipocyte stem cells with BMPs, TFGβs, FGFs, or IGF-1 augments chondrogenic potential and cell expansion; furthermore BMP6 and TGFβ3 have demonstrated chemoattractant properties in articular cartilage. Recruitment of progenitor cells and their subsequent chondrogenic differentiation may be the key in effective cartilage regeneration since homeostatic disturbance in disease cause a significant loss in the number of functional chondrocytes. Progenitor cells are found in increased numbers in OA and may include native and migrated cells from bone marrow and other joint tissues. However, despite the effort these cells are not able to overcome the present inflammatory and degradative processes found in OA joints. The expression pattern of the anabolic growth factors but also their receptors is highly regulated but also highly dynamic in healthy cartilage. Growth factor effects can be modulated by other growth factors and can be mediated by different receptor subtypes, while at the same time spatial expression prevents inappropriate exposure. The response of anabolic growth factors may also change with age, as demonstrated for IGF-1 with a decrease in production and responsiveness with increasing age. The results from the studies described in this chapter suggest the presence of an intrinsic regenerative capacity of the articular joint, which upon stimulation with appropriate anabolic factors can be reinvigorated. Resident stem cells or homing cells from adjacent tissues are able to repopulate damaged cartilage and regenerate 16 hyaline cartilage. On the other hand, the complex and dynamic pathways indicate that stimulation with growth factors alone is not sufficient for effective clinical treatment. This is underlined by demonstration of the importance of receptor subtypes, age, and posttranslational modification on growth factor responsiveness. Thus, anabolic growth factors have great potential for OA therapy because of their ability to induce chondrogenesis in diseased tissue, but unanswered questions on the timing of application, side effects, and the involvement of many other proteins have prevented it of being successful. Such a therapy will likely have to consist of a cocktail of growth factors and anti-inflammatory drugs which is designed for specific subgroups or individual patients, dependent on the patient’s age, the stage of disease, and the expression profile for the affected joint. In combination with gene therapy, to increase expression of beneficial receptors and other anabolic and anti-catabolic factors, this may result in depression of inflammation and induction of chondrogenesis. Summary and Discussion In the present thesis I have described the processes involved in the progression of focal cartilage defects to advanced osteoarthritis, a disease that can linger unnoticed for decades before exposing itself. Furthermore I have focused on the role of some of the most studied soluble mediators in cartilage regeneration, a process which appears to be latent in diseased mature cartilage but may be revived by appropriate stimulation. OA is a very common disorder of synovial joints, affecting people of all ages but is more common in the elderly [15]. It is characterized by destruction of load-bearing articular cartilage, inflammation of the synovial membrane and changes in the subchondral bone plate. OA has potentially devastating effects on the quality of life in affected individuals because of associated joint pain, restricted mobility, and the corresponding limitation of daily living activities. OA is an increasingly important public-health problem and it is expected that the incidence will increase steeply with the increasing population and fraction of elderly [24]. Healthy articular cartilage is a highly specialized avascular tissue, secreted and remodeled exclusively by chondrocytes and a population of chondrocyte progenitor cells. Cartilage homeostasis is maintained by its chondrocytes, i.e. anabolic and catabolic factors, anti-inflammatory and pro-inflammatory components are kept in a highly balanced state. Damage can be initiated via several mechanisms and factors. Traumatic injury to cartilage or ligaments, changes in subchondral bone, age-relate wear and tear, age related changes in protein functionality, obesity, diabetes, congenital joint disorders, and syndromes that cause joint instability are all factors that predispose to onset of OA [45]. These different and often unknown etiologies and driving factors make OA very hard to treat effectively. Pathological changes in load distribution and alterations in biochemical pathways cause disruptions in joint homeostasis which results in an equilibrium shift towards an inflammatory catabolic state. First changes after traumatic impact are observed within hours. Increased expression of inflammatory cytokines like TNFα, IL-1β, and IL-6 mediate upregulation of catabolic mediators and suppress Sox9-induced anabolic activity. Cartilage is 17 slowly degraded while at the same time matrix synthesis is decreased. At this early stage some chondrocytes will also respond by increasing proliferation and increasing matrix synthesis to repair cartilage but in most cases this attempt will fail. This will cause a slow destruction of articular cartilage. At the same time the release of cytokines and matrix fragments causes synovitis and changes in subchondral bone remodeling. Synovitis and bone remodeling are important driving factors in disease progression. OA has long been called a noninflammatory disease but this view is slowly changing as more evidence on involvement of inflammatory processes is found. This is underlined by the role of synovitis in OA but also the early involvement of Toll-like receptors and the complement system [45]. Up to this point, which may take years, the cartilage matrix has been progressively degraded but the collagen network is still relatively intact. It is thought that if disease progression is halted at this point, the chondrocytes are able to compensate for the matrix loss and fully regenerate. When the collagen network is damaged, many researchers tend to speak of having reached the ‘point of no return’. Whether this infamous timepoint really exists remains to be established. It is not unlikely however that at some point in disease progression the joint homeostasis is so out of balance that the chance of intrinsic repair is negligible [23]. Nonetheless, there is progressive loss of collagen, fuelled by a vicious cycle of degradation followed by increased release of catabolic factors, which in turn leads to accelerated degradation. Over time, the loss of cartilage leads to problems with joint movement due to increased friction. Disruptions in innervated tissues like the synovium and subchondral bone may also cause joint pain [22]. Without proper treatment OA may in some cases lead to total loss of cartilage which ultimately requires joint replacement. Interestingly, progression to severe OA requiring joint replacement is observed in only a small number of OA patients, suggesting a yet unknown differentiating mechanism [22]. Intrinsic repair capacity of articular cartilage has long been described as being insufficient. In part this is true, witnessing the progressive cartilage destruction in OA. However, research has shown that pathways involved in early chondrogenesis are still functional, albeit in a different role, in mature cartilage, and that by stimulation with growth factors the chondrogenic potential could be reinstated or augmented. Especially the transcription factor Sox9 appears to play an important role in inducing repair and maintaining a healthy cartilage homeostasis [75]. Anabolic growth factors like TGFβ1, BMP-2, IGF-1, and FGF-2 all, at least in part, are able to stimulate cartilage formation via Sox9 by increasing cell survival, matrix production, and suppressing catabolic factors. These factors not only affect chondrocytes but are also able to induce chondrogenesis in resident chondrocyte progenitors, bone marrow stem cells, and adipose derived stem cells [92, 93, 100]. The chondrogenic capacity of progenitor cells opens new possibilities in regenerative therapies. These undifferentiated cells have shown to be able to induce regeneration in full thickness defects in animal models which is even increased when Sox9 is overexpressed [77]. Although recent research on cartilage regeneration has delivered promising results and numerous potential pharmacological targets for therapy, the road to clinical application is still a long one. In part this is due to the 18 seemingly infinite number of experimental models which make it difficult to compare studies and even harder to draw conclusions that reach outside of the scope of specific studies. First of all a wide variety of animal models is used, ranging from transgenic mice and rats to rabbits, dogs, sheep, goats, and horses. Within these animals there are numerous methods of inducing OA-like lesions. Surgical induction of cartilage damage is done by drilling, biopsy punches, cutting, or scraping. Mechanical ways of inducing damage are also intra articular fracturing, impact loading, and meniscectomy to create joint instability. Damage can also be induced by intra articular injection of IL-1β or by a high-fat diet in transgenic mice. Each method causes different wounds which of course reflect the multifactorial disease that is OA, but makes it hard to compare. When cartilage regeneration is studied in vivo, the joint is damaged and, in many cases, attempts at regeneration are started immediately and in the same procedure. This is highly unrealistic since in the clinic OA is often diagnosed and treated years or decades after initial damage the joint. This is relevant because the degree of change in the cartilage homeostasis over time is indicative for the repair potential. Furthermore, animals involved in these studies are most likely young mature specimens while in humans OA is more likely to occur in older individuals. This is important for treatment because it is known that the severity of OA increases with age and that in e.g. rats, primates, and humans it has been demonstrated that anabolic growth factor production and responsiveness decreases with age. This suggests that regenerative results obtained in younger animal test subjects may not be relevant for the large group of elderly with OA. Another problem with the use of animals in general is that subtle differences exist in protein expression and in protein function [106]. Besides in vivo studies, in vitro research has provided large amounts of basic knowledge on molecular pathways involved in cartilage destruction and regeneration. The problem with in vitro research in general is that tissue or cells are isolated from their native environment while we try to extrapolate these data to whole tissue native conditions. Cartilage cannot be seen as an independent tissue inside the joint, but is rather the result of complex interplay between various joint tissues but also systemic factors. This is underlined by the observation that cartilage gene expression patterns may be changed upon explantation [106], much like any traumatic injury would. Chondrocytes for in vitro study can be obtained from various sources, most studies use surgical waste material, organ donors, post mortem samples, or abattoir waste. Surgical waste cartilage is often obtained after total arthroplasty, which is a procedure mostly limited to the elderly population. Other sources are post mortem cartilage which occasionally is isolated 48 hours after death. Chondrocytes isolated from these tissues are most likely a mix of healthy and diseased cells and may display age related changes as described previously. After isolation, cells are classically plated and cultured on flat polystyrene surfaces, but as previously stated, cellular behavior depends on its environment, its ‘context’. This important fact was first observed over 15 years ago when the differences in cell behavior between 2D and 3D cultures were demonstrated. These results showed the importance of tissue phenotype dominance over the cellular genotype, i.e. the ECM modulates tissue homeostasis and may regulate cell growth, survival, and differentiation [117]. This has also been 19 observed in chondrocyte culture. Chondrocytes cultured in monolayers have an unstable phenotype which leads to dedifferentiation into a fibroblast-like cell type. This is mainly due to down-regulation of Sox9, collagen type 2 and aggrecan expression [118, 119]. Because of this unstable phenotype, several culture systems have been developed to maintain chondrocytes in vitro, many of which involve 3D culture. These 3D culture systems more closely mimic the native chondrocyte environment and may provide structural support and allow for the important cell-matrix interactions. 3D culture methods like micromass- and pellet culture, but also the use of alginate beads or other scaffolds considerably increase Sox9 expression and cartilage matrix formation [119]. It has become evident from in vivo and in vitro observations that the key in maintaining or restoring chondrocyte phenotype is its environment. Whether it is in healthy or diseased cartilage, in 3D or 2D cultures, the state of the matrix and the availability of growth factors dictate chondrocyte behavior. Maintenance of Sox9 expression is especially important for the chondrocyte phenotype, and a successful regenerative therapy may only be realized when appropriate expression of this transcription factor is secured. Several pathways leading to its activation have been identified in vivo and in vitro and include the previously mentioned growth factors TGFβ1, BMP-2, IGF-1, and FGF-2. (Pre-)Clinical application of these growth factors for cartilage regeneration is limited to several phase I/II trials, and their effectiveness so far remains to be proven [25]. Although animal- and in vivo-studies show great potential for these anabolic factors, in my opinion it is unlikely that treatment with a single growth factor will lead to an effective treatment in the majority of OA cases. Early lesions in young individuals might benefit from such a therapy since the cartilage matrix and other joint tissues are relatively unaffected, and by increasing the chondrogenic capacities of resident chondrocytes but also CPCs, the homeostasis and matrix may be restored before progression into OA. However, treatment of advanced OA may require other strategies. Extensive matrix destruction, total loss of homeostasis, and age related changes in gene expression and protein functionality are some of the difficulties needed to overcome. The overall complexity of OA will likely require a combination of anti-inflammatory drugs, a cocktail of anabolic factors, gene therapy, and tissue engineering. Tissue engineering has been proposed as an alternative to current MACI procedures which require the sacrifice of ‘non-load bearing’ cartilage. In tissue engineering, autologous stem cells with chondrogenic capacities are seeded onto biodegradable scaffolds and stimulated with anabolic factors to induce cartilage formation in vitro. Acellular scaffolds may also be implanted directly and depend on recellularization and tissue formation in vivo by resident chondrocytes [31]. When loaded with growth factors these scaffolds can induce or augment intrinsic matrix deposition and reduce inflammation. Gene transfer may be a highly effective way to deal with (age related) changes in gene expression and protein functionality. Bone marrow stem cells may be isolated and altered by gene transfer before being re-implanted, or target cells are manipulated in vivo by the use of viral vectors [120]. In a rabbit OA model, Sox9 transduced bone marrow stem cells were seeded onto a degradable scaffold and implanted into a cartilage defect and showed improved short-term repair when compared to MSCs without Sox9 transduction. Temporary overexpression of Sox9 by these cells leads to induction of chondrogenesis [77]. Gene transfer as a therapy in general has great potential to overcome the 20 limitations of the current treatments in countless diseases. However, almost 70 years after its initial discovery this has only led to a handful of successful clinical applications, in part because of safety concerns due to potentially lethal side effects [120]. In conclusion, OA is an increasing problem in orthopedics. Many factors may contribute to its development, including mechanically induced impact trauma, normal wear and tear, joint instability, but also complex age related changes and obesity. Lesions often start as a focal defect, and due to changes in homeostasis and the progressive degradation of cartilage matrix often progresses into OA. Cartilage damage causes changes in chondrocyte expression pattern that lead to increased catabolic- and decreased anabolic-processes. A lot is known about disease pathogenesis, but this has not yet resulted in an effective clinical therapy to halt OA progression and induce regeneration. Over the last years, cartilage regeneration has gained increasing attention and this has led to the identification of soluble mediators and CPCs with chondrogenic capacities in native cartilage. The combination of anabolic growth factors and stem cells has demonstrated great regenerative potential in vivo and in vitro. These observations might prove crucial in the development of new, improved OA therapies. Several challenges however remain to be solved. The underlying cause of OA can be one of many and disease progression is also dependent on many different factors including the patient’s age and lifestyle. An effective therapy will have to take into account patient specific expression profiles (‘OA phenotype’) of affected joints like e.g. relevant receptors and other anabolic and anti-catabolic factors. Based on these results, a therapy can be designed for specific subgroups or individual patients. A regenerative response to OA damage is closer than ever in this era of tissue engineering and rapid technological innovations. However, further understanding of the complex pathways that initiate and lead to progression of OA, separated by spatial and temporal expression, is necessary to improve clinically relevant OA models. 21 References [1] Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect 2005;54: 465-80. [2] Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage 2007;15: 35-47. [3] Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage 2002;10: 564-72. [4] Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci 2004;117: 889-97. [5] Mollenhauer JA. Perspectives on articular cartilage biology and osteoarthritis. Injury 2008;39 Suppl 1: S5-12. [6] Pavlovich RI, Lubowitz J. Current concepts in synovial tissue of the knee joint. Orthopedics 2008;31: 160-3; quiz 164-5. [7] Iwanaga T, Shikichi M, Kitamura H, Yanase H, Nozawa-Inoue K. Morphology and functional roles of synoviocytes in the joint. Arch Histol Cytol 2000;63: 17-31. [8] Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6: 625-35. [9] Funck-Brentano T, Cohen-Solal M. Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev 2011;22: 91-7. [10] Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 1986: 34-40. [11] Lyons TJ, McClure SF, Stoddart RW, McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord 2006;7: 52. [12] Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res 2009;27: 1347-52. [13] Moskowitz RW. The burden of osteoarthritis: clinical and quality-of-life issues. Am J Manag Care 2009;15: S223-9. [14] Bitton R. The economic burden of osteoarthritis. Am J Manag Care 2009;15: S230-5. [15] Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum 1990;20: 42-50. [16] Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 2004;32: 211-5. [17] Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 2007;14: 177-82. [18] Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002;18: 730-4. [19] de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, Mastbergen SC. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage 2012;20: 846-53. [20] Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull 2008;87: 77-95. [21] Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 2003;85-A Suppl 2: 58-69. [22] Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365: 965-73. [23] Saris DB, Dhert WJ, Verbout AJ. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br 2003;85: 1067-76. [24] Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377: 2115-26. 22 [25] Berenbaum F. Targeted therapies in osteoarthritis: a systematic review of the trials on www.clinicaltrials.gov. Best Pract Res Clin Rheumatol 2010;24: 107-19. [26] Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331: 889-95. [27] Memon AR, Quinlan JF. Surgical treatment of articular cartilage defects in the knee: are we winning? Adv Orthop 2012;2012: 528423. [28] Jacobi M, Villa V, Magnussen RA, Neyret P. MACI - a new era? Sports Medicine, Arthroscopy, Rehabilitation, Therapy & Technology 2011;3: 10. [29] Simonian PT, Sussmann PS, Wickiewicz TL, Paletta GA, Warren RF. Contact pressures at osteochondral donor sites in the knee. Am J Sports Med 1998;26: 491-4. [30] Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009;4: 324-35. [31] Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res 2012;347: 613-27. [32] Dye SF, Wojtys EM, Fu FH, Fithian DC, Gillquist I. Factors contributing to function of the knee joint after injury or reconstruction of the anterior cruciate ligament. Instr Course Lect 1999;48: 18598. [33] Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum 2000;43: 215-25. [34] Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology 2004;41: 479-91. [35] Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res 2001;19: 703-11. [36] Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am 1977;59: 1068-76. [37] Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys 1995;322: 87-96. [38] Borrelli J, Jr., Ricci WM. Acute effects of cartilage impact. Clin Orthop Relat Res 2004: 33-9. [39] Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat 2005;187: 473-85. [40] Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, Guilak F, Olson SA. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res 2011;29: 501-10. [41] Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem 2004;279: 19502-11. [42] Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther 2010;12: R229. [43] Lohmander LS, Hoerrner LA, Lark MW. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum 1993;36: 181-9. [44] Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage 2007;15: 719-34. [45] Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 2012;4: 269-85. [46] Fichter M, Korner U, Schomburg J, Jennings L, Cole AA, Mollenhauer J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J Orthop Res 2006;24: 63-70. [47] Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis 2010;2: 349-59. 23 [48] Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012;51: 249-57. [49] Bradley K, North J, Saunders D, Schwaeble W, Jeziorska M, Woolley DE, Whaley K. Synthesis of classical pathway complement components by chondrocytes. Immunology 1996;88: 648-56. [50] Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, Millett PJ, Lee DM. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther 2007;9: R36. [51] Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, Crish JF, Bebek G, Ritter SY, Lindstrom TM, Hwang I, Wong HH, Punzi L, Encarnacion A, Shamloo M, Goodman SB, Wyss-Coray T, Goldring SR, Banda NK, Thurman JM, Gobezie R, Crow MK, Holers VM, Lee DM, Robinson WH. Identification of a central role for complement in osteoarthritis. Nat Med 2011;17: 1674-9. [52] Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol 2009;46: 830-9. [53] Botter SM, van Osch GJ, Waarsing JH, Day JS, Verhaar JA, Pols HA, van Leeuwen JP, Weinans H. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology 2006;43: 379-88. [54] Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol 2003;15: 628-33. [55] Westacott CI, Webb GR, Warnock MG, Sims JV, Elson CJ. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum 1997;40: 1282-91. [56] Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2005;13: 988-97. [57] Prasadam I, Friis T, Shi W, van Gennip S, Crawford R, Xiao Y. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone 2010;46: 226-35. [58] Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, CreightonAchermann L, Lotz M. The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis Rheum 2001;44: 2768-76. [59] Kwan Tat S, Amiable N, Pelletier JP, Boileau C, Lajeunesse D, Duval N, Martel-Pelletier J. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford) 2009;48: 1482-90. [60] Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factorbeta/bone morphogenic protein signalling. Arthritis Res Ther 2007;9: R100. [61] Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest 2008;88: 264-74. [62] Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 2010;12: 211. [63] Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 2002;46: 2648-57. [64] de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 2012. [65] Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 1997;24: 365-71. [66] Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005;64: 1263-7. 24 [67] Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7: 33-42. [68] McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol 2001;13: 534-40. [69] Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, Needham MR, Read SJ, Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage 2009;17: 464-72. [70] Swingler TE, Wheeler G, Carmont V, Elliott HR, Barter MJ, Abu-Elmagd M, Donell ST, BootHandford RP, Hajihosseini MK, Munsterberg A, Dalmay T, Young DA, Clark IM. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum 2012;64: 1909-19. [71] Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One 2008;3: e3740. [72] Vasara AI, Hyttinen MM, Pulliainen O, Lammi MJ, Jurvelin JS, Peterson L, Lindahl A, Helminen HJ, Kiviranta I. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage 2006;14: 1066-74. [73] Geyer M, Borchardt T, Schreiyack C, Wietelmann A, Muller-Schrobsdorff F, Muller C, MullerLadner U, Dinser R. Endogenous regeneration after collagenase-induced knee joint damage in the adult newt Notophthalmus viridescens. Ann Rheum Dis 2011;70: 214-20. [74] Cosden RS, Lattermann C, Romine S, Gao J, Voss SR, MacLeod JN. Intrinsic repair of fullthickness articular cartilage defects in the axolotl salamander. Osteoarthritis Cartilage 2011;19: 2005. [75] Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res 2012;27: 2511-25. [76] Salminen H, Vuorio E, Saamanen AM. Expression of Sox9 and type IIA procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum 2001;44: 947-55. [77] Cao L, Yang F, Liu G, Yu D, Li H, Fan Q, Gan Y, Tang T, Dai K. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011;32: 3910-20. [78] Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun 2007;358: 99-103. [79] Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem 2012;151: 247-54. [80] Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol 2004;2: e355. [81] Schmal H, Niemeyer P, Zwingmann J, Stoffel F, Sudkamp NP, Mehlhorn AT. Association between expression of the bone morphogenetic proteins 2 and 7 in the repair of circumscribed cartilage lesions with clinical outcome. BMC Musculoskelet Disord 2010;11: 170. [82] Dell'accio F, Vincent TL. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur Cell Mater 2010;20: 210-7. [83] Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FA, van den Berg WB, van der Kraan PM. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther 2007;9: R102. [84] Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res 2009;27: 602-11. [85] Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger DC, Loeser RF. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage 2007;15: 421-30. 25 [86] Chubinskaya S, Otten L, Soeder S, Borgia JA, Aigner T, Rueger DC, Loeser RF. Regulation of chondrocyte gene expression by osteogenic protein-1. Arthritis Res Ther 2011;13: R55. [87] Bobacz K, Gruber R, Soleiman A, Erlacher L, Smolen JS, Graninger WB. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum 2003;48: 2501-8. [88] Bos PK. Articular cartilage repair and the evolving role of regenerative medicine. Open Access Surgery 2010: 109. [89] Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 2009;11: R85. [90] Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 2004;50: 1522-32. [91] Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther 2011;13: R64. [92] Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 2010;376: 440-8. [93] Mendelson A, Frank E, Allred C, Jones E, Chen M, Zhao W, Mao JJ. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J 2011;25: 3496-504. [94] Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage 2007;15: 597-604. [95] Diao H, Wang J, Shen C, Xia S, Guo T, Dong L, Zhang C, Chen J, Zhao J, Zhang J. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A 2009;15: 2687-98. [96] van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest 1994;71: 279-90. [97] Cuevas P, Burgos J, Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun 1988;156: 611-8. [98] Hiraki Y, Shukunami C, Iyama K, Mizuta H. Differentiation of chondrogenic precursor cells during the regeneration of articular cartilage. Osteoarthritis Cartilage 2001;9 Suppl A: S102-8. [99] Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage 2005;13: 537-44. [100] Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrowderived mesenchymal stem cells. Tissue Eng Part A 2010;16: 1009-19. [101] Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage 2004;12: 963-73. [102] Ellman M, Yan D, Ahmadinia K, Chen D, An H, Im H. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem 2012. [103] Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A 2002;99: 8259-64. [104] Orito K, Koshino T, Saito T. Fibroblast growth factor 2 in synovial fluid from an osteoarthritic knee with cartilage regeneration. J Orthop Sci 2003;8: 294-300. [105] Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum 2009;60: 2019-27. [106] Vincent TL. Fibroblast growth factor 2: good or bad guy in the joint? Arthritis Res Ther 2011;13: 127. [107] Martel-Pelletier J, Di Battista JA, Lajeunesse D, Pelletier JP. IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res 1998;47: 90-100. 26 [108] Shakibaei M, Seifarth C, John T, Rahmanzadeh M, Mobasheri A. Igf-I extends the chondrogenic potential of human articular chondrocytes in vitro: molecular association between Sox9 and Erk1/2. Biochem Pharmacol 2006;72: 1382-95. [109] Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br 2002;84: 276-88. [110] Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 2011;6: e28663. [111] Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. J Clin Endocrinol Metab 1997;82: 1484-91. [112] Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a nonhuman primate model of naturally occurring disease. Arthritis Rheum 2000;43: 2110-20. [113] Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum 2006;54: 3850-8. [114] Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor regulation of growth factors in articular chondrocytes. J Biol Chem 2009;284: 6697-704. [115] Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A 2001;98: 160-5. [116] Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum 2008;58: 3788-97. [117] Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997;137: 231-45. [118] Kolettas E, Muir HI, Barrett JC, Hardingham TE. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology (Oxford) 2001;40: 1146-56. [119] Li H, Davison N, Moroni L, Feng F, Crist J, Salter E, Bingham CO, Elisseeff J. Evaluating Osteoarthritic Chondrocytes through a Novel 3-Dimensional In Vitro System for Cartilage Tissue Engineering and Regeneration. Cartilage 2012;3: 128-140. [120] Greenberg AJ, McCormick J, Tapia CJ, Windebank AJ. Translating gene transfer: a stalled effort. Clin Transl Sci 2011;4: 279-81. 27