N° 2- Etiopathology of chondral defects
advertisement
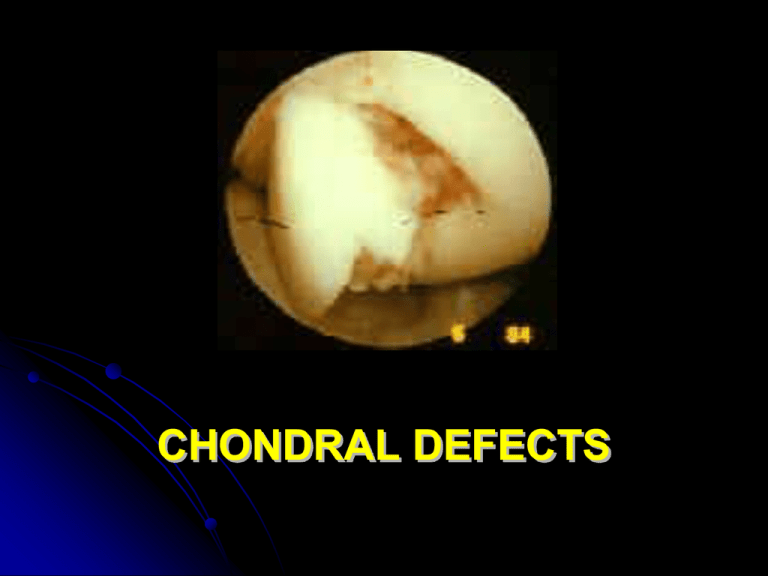
CHONDRAL DEFECTS By a retrospective review of 31,516 knee arthroscopies there were: 41 % Outerbridge Grade III chondral injuries 19.2% Outerbridge Grade IV chondral injuries (Curl W, et al. The Journal of Arthroscopies and Related Surgery 13: 456-460, 1997) Full-thickness chondral injuries, secondary to work-related and sport activities are common, with an incidence of: 5-10% of acute hemartrosis (Minas T. Instr. Course Lects. 48: 629-643, 1999) CHONDRAL DEFECTS CAUSES mechanical (trauma, instability) degenerative/inflammatory (OA/RA) Mechanical insult such as high intensity impact or torsional loading of a joint premature wear-and-tear of the cartilage that "cushions" the bone surfaces in a joint. the decline of the chondrocyte synthetic response and the progressive loss of tissue ( osteoarthrosis, also referred to as osteoarthritis, degenerative osteoarthritis, degenerative joint disease) disruption or alteration of cartilage matrix the chondrocytes response to tissue damage (anabolic and catabolic activity as well as proliferation of chondrocytes) ARTICULAR CARTILAGE LESIONS Cartilage has limited selfrepair capabilities articular cartilage defects will ultimately result in chronic tissue loss OUTERBRIDGE classification documents the progression of cartilagineous lesions: Grade 0: normal articular cartilage Grade I: softening and swelling of articular cartilage Grade II: partial thickness (fissuring on the surface) Grade III: fissuring to the level of subchondral bone Grade IV: subchondral bone exposed COMMON SYMPTOMS: the joint cannot handle a load as heavy as before difficulty in flexing or straightening completely the joint Swelling/irritation/pain and "heating up“ the joint seems to get better for a while before the symptoms simply reappear later Partial/full giving way TREATMENT OPTIONS Symptomatic/functional (arthroscopic lavage with debridement) drugs (chondroprotectors, NSAIDS, steroids) rehabilitation TKA - MKA Effective repair (surgical techniques) cell based repair/regeneration CELL-BASED REPAIR: OPTIONS bone marrow stimulation techniques perichondrial and periosteal grafts osteochondral autografts (mosaicplasty/OATS) osteochondral allografts (massive reconstr.) autologous cartilage implantation (A.C.I.) tissue engineering (HYALOGRAFT® C) BONE MARROW STIMULATION TECHNIQUES the sub-chondral bone is penetrated to reach a area of vascularization, stimulating the formation of a fibrin clot containing pluripotent stem cells able to reproduce – and by fibrous metaplasia- to realize a riparative fibrocartilageous tissue BONE MARROW STIMULATION TECHNIQUES drilling (Pridie,1959) - to promote the ingrowth of blood vessels and mesenchymal stem cells - decompression of subchondral cancellous bone intraosseous pressures immediate pain relief (transient effect due to closure of drill holes by fibrous tissue) BONE MARROW STIMULATION TECHNIQUES microfracture (Steadman, 1995) - to break through the superficial subchondral bone abrasion arthroplasty (Johnson, 1984) - abrasive removal of the superficial subchondral bone DRAWBACKS uncertain outcomes unpredictable short term results developing of fibrocartilage that lacks the durability and many of the mechanical properties of the hyaline cartilage Deep holes/ fractures may weaken the subchondral layer such an extent that the bone is depressed leading to articular incongruity (Insall, 1974) OSTEOCHONDRAL AUTOGRAFTS OR MOSAICPLASTY / OATS (Hangody, 1991) OSTEOCHONDRAL AUTOGRAFTS (Hangody, 1991) Osteochondral plugs are transferred from a non load bearing area of the joint and implanted as a mosaic by a press-fit technique into the drill holes in the recipient area of the defect by arthroscopically or arthrotomically procedure A composite cartilage surface consisting of 70% to 90% transplanted hyaline cartilage and 10% to 30% of integrated fibrocartilage Fibrocartilage fills the spaces between the transplanted grafts and eliminates the incongruities of the surface – THE TRANSPLANTED HYALINE CARTILAGE IS LINKED TO SUBCHONDRAL BONE ADVANTAGES STABLE CONNECTIONS BETWEEN CARTILAGE AND BONE IS PRESERVED – CANCELLOUS BONE HEALS VERY RAPIDLY 6 ms DRAWBACKS complex surgical procedure (meticolous attention to matching size and inlay fixation) violation of subchondral bone ambiguous indications limited application of one/few plug/s for chondral lesion lesser than 2-4 cm2 to avoid significative ratio of fibrocartilage tissue donor site morbidity not reproducible Importance of the congruity of the graft surface to avoid its degeneration (Lindholm) THE BIOLOGICAL PARADOX Cartilage tissue will not repair, however chondrocytes grow ex vivo A.C.I. (Peterson) injection of cell suspension seal tight coverage with periosteal flap A.C.I.Autologous cartilage transplantation (source: http://www.genzyme.com/carticel) > 4000 patients treated in the world good-excellent results at 3 yrs follow-up in 85% of cases A.C.I. INDICATIONS Lesion size 2cm2 patient expectations with regard to expected level of activity – high/low demand sports – daily living activities surgical invasiveness compliance with rehabilitation procedural costs A.C.I. indications - lesion size 2 cm2 for a low demand patient Small focal defects ( 2 cm) when treated with debridement or marrow stimulation techniques Have not been shown to progress to DJD (Homminga 1990; Brittberg 1994; Hubbard 1996; Messner 1996) Due to the fact that the chondral borders efficaciously SHOULDER the exposed bone from damaging the opposing articular surface Arthroscopic debridment offers symptomatic relief for up to 5 years in 50% of patients, with a 50% success returning to sports and 75% success of being confortable with activities of daily living is a minimally invasive procedure with low cost treatment A.C.I. Indications lesion size 2 cm2 for a high demand patient Mosaicplasty, although converts a chondral to an osteochondral injury – minimally invasive – low cost A.C.I. when other techniques fail, has been shown to be 90% successful in a revision chondral surgery situation to return a patient to a high level activity A.C.I. indications - lesion size 2 cm2 In large ( 2-3 cm2) chondral defects, the repair tissue must prevent the subchondral bone from bottoming out, so that secondary articular damage does not occur to the opposing articular surface DJD A.C.I. is the first line treatment as this A durable repair tissue with > 90% success Large osteochondral allografts (?) If A.C.I. fails TKA, MKA A.C.I. Indications Tibio-femoral malalignment Patello-femoral malalignment ACL insufficiency bone insufficiency (osteochondritis dissecans, avascular necrosis, osteochondral fracture) Must be managed PRIOR to, or CONCURRENTLY managed with, the chondral injury A.C.I.- limitations problematic handling of coltures in suspension complicated surgical procedure chondrocyte de-differentiation (cells acquire a less specialized phenotype and loose the capacity to produce essential molecules : chondrocytes assume a fibroblastoid morphology and cease the production of molecules typical of hyaline cartilage) TISSUE ENGINEERING EMPLOYS LABORATORY GROWN TISSUES FOR THERAPEUTIC USES Cartilage has limited self-repair capabilites articular cartilage defects will ultimately result in chronic tissue losses To contrast this relentless outcome new reconstructive techniques have been developed such as autologous cultured chondrocyte implantation (ACCI) medium-term results are encouraging but with limitations problematic handling of cultures in suspension complicated surgical procedure chondrocyte de-differentiation: cells acquire a fibroblastoid phenotype and loose the capacity to produce the typical ECM molecules able to regenerate a physiological-like tissue Hyaluronan-based Tissue Engineering Use of biomatherials derived from jaluronic acid (Hyaff-11) Ideal three-dimensional scaffold for the cultivation of human autologous chondrocytes 1. Ability to restore the differentiated phenotype maintaining chondrocyte capability to produce collagen type II (hyaline cartilage marker) 2. Improved and simplified implant technique chondrocytes assume a cease the production fibroblastoid morphology of collagen type II typical of fibrocartilage 3 days 14 days 24 hrs 14 days DAY 50 grafting on patient DAY 0 DAY 28 cartilage biopsy (150 mg) cartilage formation in vitro culture of chondrocytes DAY 14 Seeding on HYAFF® scaffold FAB s.r.l APPROACH Autologous Chondrocytes + Tridimensional hyaluronan scaffold BIO-ENGINEERED TISSUE Hyalograft-C: technical advantages easy to handle and surgically apply conformable may be cut to fit defect no need for seal tight periosteal coverage Hyalograft-C: indications and use • Symptomatic/ asymptomatic defects of femoral condyle, knee cap and tibial plateau caused by acute or recurred injures Procedure Arthroscopical taking of a cartilage biopsy (150 mg) Hyalograft-C: indications and use Pregrafting surgical curettage of the lesion Application of graft in miniarthrotomy or arthrotomy Secured to the defect by applying a periostal flap sutured at the edges of the lesion Hyalograft-C: indications and use Post-surgical treatment: at surgeon’s discretion an articular drainage and a compressive bandage should be applied for 24 hours post grafting articular movement forbidden for 24 hours postgrafting early postsurgical passive rehabilitation therapy is advisable Hyalograft-C: indications and use postsurgical passive rehabilitation : Start with a passive rehabilitation with an excursion of 0°40°C and perform isometric exercises for quadriceps Articular weight bearing gradually applied from the third week From 6-12 weeks increasing the weight bearing during walking with crutches. Moderate walking, cyclette and swimming depending on the state of the knee. From the third month simulation of normal activity if no pain is present.