Ch6 Part II Solutions and biological molecules Answer Section
advertisement

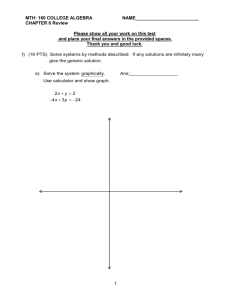
Ch6 Part II Solutions and biological molecules True/False Indicate whether the statement is true or false. ____ 1. Water is a polar substance. ____ 2. In water, the oxygen is more negative because electrons spend more time around it. ____ 3. Water is considered the universal solvent because it can even dissolve lipids. ____ 4. Kool-aid is a solution and water is the solute. ____ 5. When salt (NaCl) is dropped into water the Na is pulled to the oxygen part of water breaking salt apart. ____ 6. A mixture is a combination of 2 or more substances that chemically combine. ____ 7. A heterogenous mixture is uniform throughout. ____ 8. A solvent is defined as what the solute dissolves in. ____ 9. A colloid is a solution whose particles don’t settle out. ____ 10. Hydroxide ions, OH- is what determines an acid. Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 11. When added to water an acid is a substance that releases which of the following into solution. a. hydroxide ions c. Hydrogen ions b. Negative ions d. positive ions ____ 12. What happens when you mix a strong acid with a strong base? a. You still get an acid because acids are c. a neutral solution of water and salt stronger b. You get a base because a base has a high d. a salt solution slightly basic pH ____ 13. A pH buffer does what? a. controls the pH level when too acidic or c. keeps solutions basic too basic b. keeps solutions acidic d. keeps solution alkaline ____ 14. Which of the following would be a good reason to maintain a certain pH in the body? a. all of the body in mostly a basic pH and c. The body operates mainly in the acidic should be kept basic range and should be kept acidic b. Various parts of the body operate at d. the body can operate in any pH range various pH levels and needs to adjust so as not to be too acidic or too basic. ____ 15. Which of the following elements is neccessary to be an organic molecule? a. nitrogen c. hydrogen b. oxygen d. carbon ____ 16. Organic molecules only have a few elements that can they can form from. Which of the following accounts for the diversity of organic molecules and their functions? a. the ability to form so many different kinds c. forming different shapes even with the of bonds same elements b. forming small molecules d. all the above ____ 17. Which of the following bonds does an organic compound not form? a. covalent c. single carbon bonds b. ionic d. double carbon bonds ____ 18. Which element would need to be removed from the molecule in Figure 6-2 to make it unsaturated? ____ 19. ____ 20. ____ 21. ____ 22. ____ 23. ____ 24. ____ 25. ____ 26. ____ 27. ____ 28. Figure 6-2 a. carbon c. oxygen b. hydrogen d. phosphorus A student set up four test tubes containing a carbohydrate solution in which to perform carbohydrate digestion. Supplies included amylase (enzyme that breaks down carbs) and an incubator. In which tube listed would carbohydrate digestion proceed most quickly? a. Tube 1: No amylase, room temperature (25°C) b. Tube 2: No amylase, body temperature (37°C) c. Tube 3: Amylase present, room temperature (25°C) d. Tube 4: Amylase present, body temperature (37°C) Which property is responsible for the fact that water and oil do not mix? a. density c. phase b. mass d. polarity Which is the best example of a solution and not a colloid a. blood c. paint b. milk d. salt water When added to water, how does an acid affect the pH and H+ concentration? a. Both pH and H+ decrease. b. Both pH and H+ increase. c. The pH decreases while the H+ increases. d. The pH increases while the H+ decreases. Which element is found in proteins but not carbohydrates or lipids? a. C c. N b. H d. O In humans and other multicelluar organisms, which substance plays a central role as a primary energy source? a. carbohydrate c. protein b. fat d. water Glycogen, used to store energy in the liver and muscle tissue, is an example of which type of molecule? a. carbohydrate c. saturated fatty acid b. protein d. steroid Amino acids are the building blocks of which macromolecule? a. carbohydrate c. lipid b. DNA d. protein An organism’s genetic information is stored in which type of macromolecule? a. DNA c. lipid b. carbohydrate d. protein Which of the following is not found in a fat? ____ 29. ____ 30. ____ 31. ____ 32. ____ 33. ____ 34. ____ 35. ____ 36. ____ 37. ____ 38. ____ 39. ____ 40. a. a glycerol head c. an unsaturated fatty acid b. a saurated fatty acid d. nitrogen Besides energy, fats can be used for.... a. forming cell membranes with c. making cholesterole and vitamin D phospholipids b. forming steroid hormones d. all the above Which of the following is not a protein. a. sucrose c. antibodies b. hemoglobin d. collagen and elastin Which of the following is less likely to unfold regarding protein structure. a. primary folding c. tertiary folding b. secondary folding d. quantinary folding Besides a phosphate group and a sugar, what is the 3rd thing needed to make a nucleotide? a. glucose c. a nitrogen base b. a nucleic acid d. none of these The sugar that forms a nucleotide is.... a. Ribose c. fructose b. Glucose d. sucrose How many different nucleobases are there to form DNA, a nucleic acid. a. 1 c. 3 b. 2 d. 4 Which of the following is a difference between RNA and DNA a. RNA is a single strand of nucleotides c. DNA has no oxygen b. DNA is a double strand d. all the above Sucrose is a... a. monosaccharide b. disaccharide Plants use polysaccharides to form.... a. cellulose b. chitin Why do lipids make a good cell membrane? a. they allow polar substances in and out b. they dont break down in water Functional proteins are also known as.... a. fibrous proteins b. globular proteins Nucleic acids, carbohydrates and proteins can also be refered to as... a. polymers c. macromolecules b. long chains of monomeres d. all the above Ch6 Part II Solutions and biological molecules Answer Section TRUE/FALSE 1. ANS: T This is the correct definition. PTS: 1 TOP: 6-5 2. ANS: T This is true DIF: Bloom's Level A | DOK 1 REF: 156 PTS: 1 NAT: LS_1b 3. ANS: F false, it cannot. DIF: Bloom's Level C | DOK 1 TOP: 6-6 REF: 158 PTS: 1 DIF: Bloom's Level D | DOK 1 NAT: LS_1b TOP: 6-6 4. ANS: F This is false, water is the solvent REF: 159 PTS: 1 TOP: 6-7 5. ANS: T true DIF: Bloom's Level A | DOK 1 REF: 160 PTS: 1 DIF: Bloom's Level C | DOK 2 TOP: 6-8 6. ANS: F false, dont chemically combine. REF: 161 PTS: 1 TOP: 6-10 7. ANS: F false is not uniform REF: 165 PTS: 1 8. ANS: T true PTS: 1 9. ANS: T true PTS: 1 10. ANS: F false, H ions DIF: Bloom's Level D | DOK 1 PTS: 1 MULTIPLE CHOICE 11. 12. 13. 14. 15. 16. 17. 18. ANS: C PTS: 1 ANS: C PTS: 1 ANS: A PTS: 1 ANS: B PTS: 1 ANS: D PTS: 1 ANS: C PTS: 1 ANS: B PTS: 1 ANS: B Two atoms of hydrogen would need to be removed. Feedback A B C D Removal of a carbon atom would break the chain. Yes, removing hydrogen atoms would leave a double bond. Oxygen atoms are not involved here. There is no phosphorus in this molecule. PTS: 1 DIF: Bloom's Level C | DOK 3 REF: 169 TOP: 6-13 19. ANS: D Starch digestion would proceed most quickly in the presence of amylase at body temperature. Feedback A B C D An enzyme would increase the rate of the reaction. An enzyme would increase the rate of the reaction. The reaction would proceed more quickly at body temperature. Well done. PTS: 1 DIF: Bloom's Level F | DOK 3 TOP: 6-7 20. ANS: D Water is polar while oil is nonpolar. REF: 159 Feedback A B C D This explains why oil floats on water, but not why they do not mix. Mass of oil molecules is only slightly related to this idea. Both substances are liquid. Correct. PTS: 1 DIF: Bloom's Level D | DOK 2 REF: 163 TOP: 6-8 21. ANS: D A solution is a homegeneous substance that contains a solute dissolved in a solvent. Feedback A B C D This is a colloid. See page 163. This is a colloid. Well done. PTS: 1 DIF: Bloom's Level D | DOK 2 TOP: 6-9 22. ANS: C The pH decreases as the H+ increases. REF: 163 Feedback A B C D Only pH decreases. The pH would decrease. Correct. This is opposite of the correct answer. PTS: 1 DIF: Bloom's Level D | DOK 2 TOP: 6-10 23. ANS: C Proteins have nitrogen, but carbohydrates and lipids do not. REF: 164 Feedback A B C D All have C. All have H. Correct. All have O. PTS: 1 DIF: Bloom's Level B | DOK 1 REF: 168 NAT: LS_5b TOP: 6-12 24. ANS: A Carbohydrate is the key substance for energy metabolism. Fat is used for storage but not typically as a direct energy source. Feedback A B C D Well done. This is mostly for energy storage, not use. This can be used for energy but that is not its main role. Water does not provide energy, though it is essential for life. PTS: 1 DIF: Bloom's Level C | DOK 2 NAT: LS_5b STA: LS.10.11 TOP: 6-13 25. ANS: A Glycogen is made of glucose and is a carbohydrate. Feedback A B C Correct. See page 168. Glycogen is not a fatty acid. REF: 168 D See page 168. PTS: 1 DIF: Bloom's Level A | DOK 2 NAT: LS_5b STA: LS.10.11 TOP: 6-13 26. ANS: D Amino acids are the building blocks of protein. REF: 168–169 Feedback A B C D Carbohydrates are made of sugar units. DNA is made of nucleotides. See page 170. Correct. PTS: 1 DIF: Bloom's Level B | DOK 2 NAT: LS_5b STA: LS.10.11 TOP: 6-12 27. ANS: A DNA is the genetic material of the cell. REF: 170 Feedback A B C D 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. Correct. This provides fuel. See page 171. Protein is not the substance used to store the genetic information. PTS: NAT: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: 1 LS_5b D D A D C A D D B A B B D DIF: STA: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: Bloom's Level A | DOK 1 LS.12.1 TOP: 6-13 1 1 1 1 1 1 1 1 1 1 1 1 1 REF: 171