Name Atomic Structure Directions: Use pages 280
advertisement
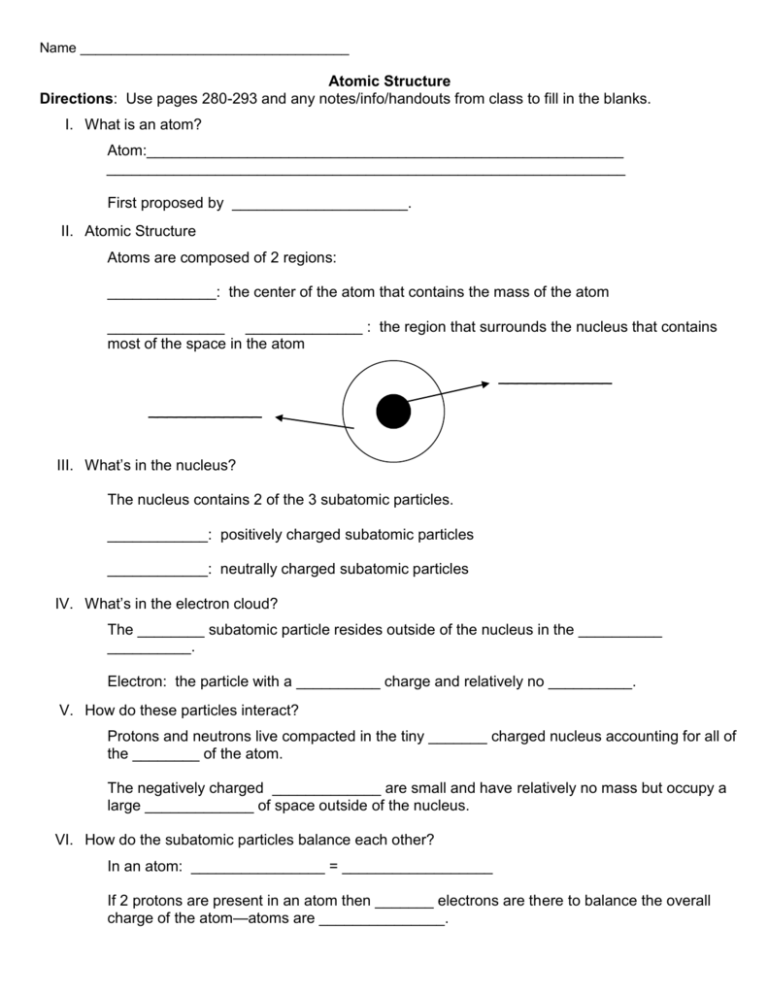
Name ___________________________________ Atomic Structure Directions: Use pages 280-293 and any notes/info/handouts from class to fill in the blanks. I. What is an atom? Atom:_________________________________________________________ ______________________________________________________________ First proposed by _____________________. II. Atomic Structure Atoms are composed of 2 regions: _____________: the center of the atom that contains the mass of the atom ______________ ______________ : the region that surrounds the nucleus that contains most of the space in the atom ____________ ____________ III. What’s in the nucleus? The nucleus contains 2 of the 3 subatomic particles. ____________: positively charged subatomic particles ____________: neutrally charged subatomic particles IV. What’s in the electron cloud? The ________ subatomic particle resides outside of the nucleus in the __________ __________. Electron: the particle with a __________ charge and relatively no __________. V. How do these particles interact? Protons and neutrons live compacted in the tiny _______ charged nucleus accounting for all of the ________ of the atom. The negatively charged _____________ are small and have relatively no mass but occupy a large _____________ of space outside of the nucleus. VI. How do the subatomic particles balance each other? In an atom: ________________ = __________________ If 2 protons are present in an atom then _______ electrons are there to balance the overall charge of the atom—atoms are _______________. The neutron has _____ charge therefore they do not have to equal the number of protons or electrons. VII. How do we know the number of subatomic particles in an atom? Atomic number: indicates the number of ___________ in an atom. Ex: hydrogen’s atomic number is 1, so hydrogen has _______ proton**. Ex: carbon’s atomic number is ______, so carbon has _____ protons**. **The number of ___________ identifies the atom 2 p = ________ 29 p = _______ VIII. How do we know the number of subatomic particles in an atom? Atomic ______ : the number (or sum) of protons and neutrons in the nucleus. Ex: helium has a mass of ________. Since it has 2 protons, it must have 2 neutrons. **number of neutrons = Atomic ________ - # of ________ IX. Determining the number of protons and neutrons: Li has an atomic mass of 7 and an atomic # of 3 Protons = 3 (same as the # of __________ ) Neutrons = 7 – 3 = 4 (Atomic _________ - # of________ ) Ne has a mass number of 20 and an atomic number of 10. Protons = _______ Neutrons = ______ X. What about the electrons? The electrons are equal to the number of ___________. So protons = __________ = __________ number Ex: He has a mass number of 4 and an atomic number of 2 p=2 n=2 e-= 2 XI. Determine the number of subatomic particles in the following: Cl has a mass number of 35 and an atomic number of 17 P = ______ n = _______ e- = ______ K has a mass number of 39 and an atomic number of 20. P = ______ n = _______ e- = ______