Acids

Acids and Bases worksheet
For more detail look at Science aspects 2 Chapter 5.4 and 5.5
Acids
Acids are substances which contain hydrogen. Importantly when they are dissolved in water to make a solution, they release hydrogen ions (H + ). These hydrogen ions are the part of an acid which does the work and provides the acids properties. Acids can be weak or strong. A strong acid releases a lot of hydrogen when it dissolves while a weak acid only releases a little bit
There are 3 main common strong acids. Hydrochloric acid, Nitric acid and Sulfuric acid. The chemical formula for these is given to the right. It will help if you know these. Examples of weak acids include ethanoic acid and citric acid.
Hydrochloric Acid
Nitric Acid
Sulfuric Acid
HCl
HNO
H
2
SO
3
4
Properties of acids
Acids have a distinct taste. In chemistry we never taste chemical compounds so in a laboratory this is never done. However many foods have acids in them. For example Lemons which contain citric acid.
Tasted a lemon in class and reported that it tastes
Bases
Bases are chemicals that dissolve in water to produce hydroxide ions (OH ).
The hydroxide ions give bases’ their properties. Bases can also be strong or weak. The strong bases are usually metal hydroxides such as sodium hydroxide which produce a lot of hydroxide ions in solution. Weak bases including ammonia and carbonates react with water to make a small amount of hydroxide ion.
Properties of bases
Bases are also present in many foods. An example of a base is quinine which is found in tonic water.
Tasted the tonic water and reported that it tastes
Concentration
Acids and bases are solutions in water. Like any solution you can increase or decrease the the amount of an acid or a base dissolved in it. The amount of an acid or base you dissolve is its concentration. The more concentrated an acid or base is the more reactive it will be. Remember when we did the reaction of sulfuric acid with sugar to make the black carbon snake. This used a concentrated sulfuric acid.
Dilute sulfuric acid would not have produced this reaction.
Indicators and pH
Indicators are chemicals which change colour when they come into contact with acids and bases. As a result they can be used to see if something is acidic or basic. Remember when we were demonstrating respiration and we burned sugar. We used an indicator bromothymol blue to test for carbon dioxide.
The carbon dioxide actually dissolved in the water to make a weak acid. This changed the solution from _____________________ to ___________________________ which indicated that the solution became acidic. There are many other indicators with their own colour changes.
A very common indicator is red and blue litmus paper.
Describe what happens to red and blue litmus paper when it is added to an acid.
Describe what happens to red and blue litmus paperwhen it is added to a base
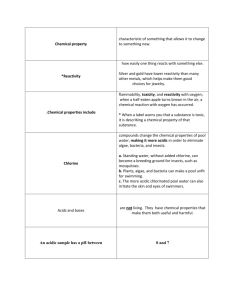
The pH (power or hydrogen) scale is a numeric scale which gives a number value to describe how acidic or basic a substance is. Water is neutral and is neither acidic nor basic. It has pH of 7. All acids have a pH value from 1-7. All bases have a pH value from 7-14.
Extra question
Summarise what you know about acids and bases in a brief concise paragraph below. Include information on tastes, pH, Indicators, concentration and acid/base strength.