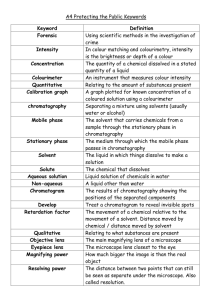
Laboratory 1
Paper Chromatography
OBJECTIVE
To use paper chromatography to separate and identify:
1) Metal ions in aqueous solution;
2) Food colours in powdered drink mixes.
BACKGROUND
The term chromatography is derived from the Greek words chroma “colour” and
graphein “to write” and was first used by the Russian botanist Mikhail Tsvet in 1900 to describe
the separation of coloured plant pigments on a column of alumina (Al 2O3). Chromatography is
an analytical technique, and it is used to separate and identify mixtures of various organic and
inorganic compounds. During a chromatographic separation the mixture is distributed between
two phases: a stationary phase and a mobile phase.
In a paper chromatography, the components in a mixture are carried along the
stationary phase, paper, at different rates by means of a moving liquid phase. This process is
referred to as “elution” and the mobile phase can be called an “eluting solvent”. The
components with lower molar masses or the less polar ones are weakly adsorbed on the paper
and will move along with the eluting solvent at a faster rate than those components of greater
molar mass or polarity. This difference in adsorption results in the separation of the
components of the mixture.
The ratio of the rate of migration of a component in a solution to the rate migration of a
component in a solution to the rate of flow of the solvent is referred to as the R f value for that
component. The Rf value may be calculated as the ratio of the distance moved by the chemical
component to that moved by the solvent since for both the component and the solvent the time
is common. Under carefully controlled conditions, the Rf value is constant for a particular
compound, solvent system, and paper.
In part A of this experiment, you will use paper chromatography to separate a mixture of
Ni2+ and Fe3+ ions. A strip of filter paper will be the stationary phase. A solution of HCl
and acetone will be the mobile phase.
Cu2+,
1
Figure 1 - Diagram of a paper chromatography set-up in solvent chamber
Small drops of solutions containing Cu2+, Ni2+ and Fe3+ ions are placed on a filter paper
strip as shown on Figure 1. The mobile phase, HCl and acetone, moves up the filter paper by
capillary action and carries the mixture of ions with it. The Ni 2+ ions tend to be strongly
absorbed onto the surface of the filter paper and so they move slowly up the paper. The Fe 3+
ions, however, are weakly absorbed onto the surface, and they move readily up the paper as
shown in Figure 1. The Cu2+ ions has absorption properties intermediate between those of Ni2+
and Fe3+. In this way, a good separation of the three ions is achieved before the solvent front
reaches the top of the paper. The actual distance that a chemical move up the stationary
phase can be used to identify the chemical. To identify a chemical, we can calculate its R f
value and compare it with those from series of known species tested under the same
conditions. If the Rf value of the unknown matches that of a known species, it is possible that
they are both the same species. The identification is confirmed by other observations,
particularly the colours and appearances of the spots. In the chromatogram of Figure 2, the R f
value of ion Cu2+ is:
Figure 2 - Calculation of Rf value of copper(II) ions.
2
Unfortunately, in this experiment, the colour of the Cu 2+ and Ni2+ ions on the filter paper
is too faint to see. In contrast, the Fe3+ ion produces a yellow colour on the filter paper and is
easy to detect. To overcome the difficulty of visualizing the non-coloured metal ions, the paper
is exposed to locating reagents that react and transform the metal ions into coloured species.
For instance, Cu2+ ion can be made more visible on the paper by reacting it with NH 3 (from
ammonia solution), to produce a compound with a deep blue colour:
Cu2+ (pale blue) + 4NH3 [Cu(NH3)4]2+ (deep blue)
Ni2+ ion can be made more visible on the paper by reacting it with dimethylglyoxime to
produce a complex compound with a bright red colour:
Once the metal ions have been transformed into coloured compounds, it is possible to
compute the Rf value of each spot.
In part B of this experiment, you will apply the technique of paper chromatography to the
separation of organic compounds in a mixture. Specifically, you will separate and identify food
colours in powdered drink mixes. As discussed for part A, by comparing both the colour and
the Rf value of each component in the test product with standard food dyes, you will be able to
identify the dye components of the product.
PROCEDURE
1. Obtain two rectangular sheets of Whatman #1 filter paper. Be careful not to touch the
paper with your fingers. Place the paper on a clean area on the bench.
2. Using a pencil, draw an "origin" line horizontally approximately 2 cm from the edge of the
paper. Locate the position where spots of each sample are to be applied by marking an X
on the line for each spot. The spots should be evenly spaced along the full width of the
chromatogram. Since the number of spots is different on each of your chromatograms the
3
spacing of the spots will be different for each of your chromatograms. Identify each spot by
writing below the line in pencil.
10 cm
3+
1, Fe
2, Cu
2+
2+
3, Ni
4, unknown
2 cm
20 cm
Figure 3 – Example of paper labeling and dimensions
Note: After the papers have been ruled for the origin lines, each student in a pair should work
independently on either Part A or Part B.
Notes:
a) It is important that you handle the filter paper at the edges with your finger so that your
fingerprints will not interfere with the chromatogram.
b) It is important that you use only pencil on the chromatogram because the colours in ink will
be carried up the chromatogram by the eluting solvent and will prevent you from locating the
spots from your samples.
Part A: Paper Chromatography of Inorganic Ions
Pour eluting solvent (HCl and acetone) into a 600 mL beaker. The level of solvent must be
approximately one half of the distance from the edge of the chromatogram to the “origin”
line. Cover the beaker with a watch glass
1. Spot each of the following samples in its proper place on the labeled chromatogram.
Spot Number
Sample
1
Iron, Fe3+
4
2
Copper, Cu2+
3
Nickel, Ni2+
4
Unknown
2. Three drops of each solution are to be applied with the tip of a glass capillary tube,
allowing time for drying after each application. Remember not to contaminate the
solutions by using the same capillary tube in more than one solution. Do not let the size
of any spot exceed 2 mm.
3. Carefully coil the paper into a cylinder with the origin line facing outwards and staple the
two ends together. The edges of the paper should not touch each other.
4. Remove the watch glass from the top of the beaker and stand the paper cylinder in the
beaker with the origin line at the bottom. Take care not to let the paper touch the glass
walls. The spots must not be immersed in the solvent. Cover the beaker with the watch
glass. Allow the solvent to rise up the paper until the “wet” mark of the solvent front is
less than 1 cm from the top of the paper
Figure 4 - Developing Chromatogram.
5. Remove the chromatogram from the beaker and immediately trace the line of the
solvent front with a pencil. This may not be a simple horizontal line and it is essential
that the position be recorded above each of the spots. It is essential that this be done
as rapidly as possible since the solvent will evaporate quickly and the “wet” mark of the
solvent front will disappear.
6. Undo the staples, spread the chromatogram flat and allow it to dry.
7. In order to locate the spots corresponding to each of the components in the solutions
the chromatogram must be “developed”.
8. Mark with a pencil the ring front of the iron ion (yellow colour).
9. In the large fume hood at the side of the room you will find containers of 15 M
ammonium hydroxide and dimethylglyoxime. Using a small piece of filter paper wet the
surface of your chromatogram with the dimethylglyoxime solution. While the
5
chromatogram is still “wet” hold it over the mouth of the ammonium hydroxide container.
Observe any changes in the appearance of the chromatogram.
10. Mark the “front” of each spot with a pencil. Remember that the unknown may contain up
to three components and that each of them may have its own spot and therefore will
need to be identified in pencil individually.
11. Pour the eluting solvent from the beaker into the appropriate waste container in the
fume hood.
Part B: Paper Chromatography of Food Dyes in Powdered Drink Mixes
The class will be provided with a series of solutions of standard food colourings. There will also
be a number of solutions of commercially available “drink” mixes. The objective is to determine
which combinations of the standard colourings were used to prepare each of the “drinks”.
12. Pour eluting solvent (ammonium hydroxide, 1-pentanol and absolute alcohol) into a 600
mL beaker. The level of solvent must be approximately one half of the distance from the
edge of the chromatogram to the “origin” line. Cover the beaker with a watch glass.
13. Prepare the chromatogram by spotting with each of the food colourings and unknown
“drinks”. Three drops of each solution are to be applied with the tip of a glass capillary
tube, allowing time for drying after each application. Remember not to contaminate the
solutions by using the same capillary tube in more than one solution. Do not let the size
of any spot exceed 2 mm.
14. Remove the watch glass from the top of the beaker and stand the paper cylinder in the
beaker with the origin line at the bottom. Take care not to let the paper touch the glass
walls. The spots should not be immersed in the solvent. Cover the beaker with the
watch glass. Allow the solvent to rise up the paper until the “wet” mark of the solvent
front is less than 1 cm from the top of the paper.
15. Remove the chromatogram from the beaker and immediately trace the line of the
solvent front with a pencil. This may not be a simple horizontal line and it is essential
that the position be recorded above each of the spots. It is essential that this be done
as rapidly as possible since the solvent will evaporate quickly and the “wet” mark of the
solvent from will disappear.
16. Undo the staples, spread the chromatogram flat and allow it to dry.
17. Mark the “front” of each spot with a pencil. Remember that each “unknown drink” may
contain multiple components and that each of them may have its own spot and
therefore will need to be identified in pencil individually.
18. Pour the eluting solvent from the beaker into the appropriate waste container in the
fume hood.
6
REPORT
Prepare a report of your experimental results by answering the questions below and using the
guidelines provided in the Chemical Student Manual.
Some reminders:
-
All measurements and calculations should be given to the correct number of significant figures
Tables and Figures must be numbered and include a descriptive caption
For all graphs, the x-y data table used to prepare the graph must be shown on the same page as the
graph
All references must be cited using ACS style (see Chemical Student Manual posted on SLATE2)
In the RESULTS and CALCULATIONS section of your report be sure to include the
following:
1. For each chromatogram:
a. Measure the distance each spot has travelled (the distance from the top of each spot to
the origin line). Measure the distance the solvent has travelled above each spot (the
distance from the solvent front to the origin line). Refer to Figure 2.
b. Record the colour of each spot.
c. Calculate the Rf value for each spot and show your calculations. Remember to show the
appropriate number of significant figures.
d. Record your observations (distances and colours) and calculated Rf values in a table.
e. Include the chromatogram in you report.
In the DISCUSSION section of your report
2. For each chromatogram, compare the Rf value, or values, of the component, or
components, in the unknown mixture, or mixtures, with the values for the known standards.
Based on these comparisons suggest the identity of the components in the unknown
mixture, or mixtures. Support your identification by other observations, especially the
colours of the spots.
3. Comment on any discrepancies between the Rf values for the knowns and unknowns (i.e.
Are there exact matches? If not, why not?).
4. If the locating reagent for Ni2+ was changed from dimethylglyoxime to CN-, such that a red
nickel(II) tetracyanide complex formed, would the Rf value for nickel change? Why?
5. If the eluting solvent was changed, would the Rf values for each metal ion change? Explain
why.
6. Why were you told to place the solvents in the beakers before you prepared the
chromatograms?
7
References
1. Nelson, J. H., and Kemp, K. C., Chemistry: The Central Science Laboratory
Experiments, 8th ed.; Prentice Hall: Upper Saddle River, N.J., 2000.
2. Brown, T.L., Bursten, B.E., LeMay, H.E., Murphy, C.J. and Woodward, P.M.
Chemistry: The Central Science, 12th ed.; Pearson Prentice Hall: United States
Of America, 2012.
8