Sealed With a Kiss chromatography lab v2
advertisement

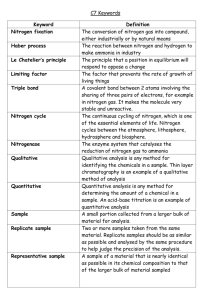
IDC4U3 Panayiotou Sealed With a Kiss Purpose: To use paper chromatography to solve a crime. Brentyn is a popular player on the LHS football team. Recently he has received several notes from a secret admirer and Brentyn would like to know who it is. All of the notes were in envelopes sealed with a smooch! Brentyn managed to get lipsitcks and lip-print samples from each of the girls he suspects. Your task is to determine which girl wrote the notes. Materials Chromatography paper Pencil Ruler Scissors 4 Cotton swabs Aluminum foil or plastic wrap 4 Lipstick samples Chromatography solvent Beaker and test tubes Evidence envelope 4 smooches Hand lens Safety You must wear goggles at all times. Dispose of the chromatography solvent in the waste disposal bin provided. Procedure: 1. To create your chromatogram obtain a piece of chromatography paper and cut it like the image shown to the left. Using a pencil draw a line on top of the V cut out. 2. Use a cotton swab to place a small line of lipstick on the line. 3. Pour a small amount of solvent in the bottom of the test tube. The solvent should be just enough to touch the chromatogram tip when you put it in but not cover the lipstick sample. 4. Carefully place the prepared chromatogram into the test tube with the pointy end in the solvent. Pass a paper clip through the top of the chromatogram and seal the test tube. It should stand alone in a beaker or test tube rack. 5. Cover the test tube with a stopper, aluminum foil or plastic wrap. Leave the test tube undisturbed until the solvent reaches the top of the chromatography paper. 6. Repeat for the other samples of lipsticks. 7. While you are waiting obtain the suspect and evidence lip prints. 8. Using a hand lens, observe each lip print. Divide each lip print into quadrants and sketch the pattern one quadrant at a time. Record your observations in Table 1. Remember always use pencil for drawings. 9. When the chromatogram is finished separating, remove the paper from the beaker and mark with pencil the solvent front (the farthest distance travelled). Allow the chromatogram to dry completely. Refer to the diagram on the right. IDC4U3 Panayiotou 10. Calculate the Rf value for each of the samples and record your data in Table 2. To calculate Rf values: measure the distance in millimeters from the line of origin (pencil line) to the sample front. Then measure the distance of the solvent front from the original depth of the solvent. 11. Use the formula below to calculate Rf values for each sample. Rf = Distance travelled by the sample (mm) Distance travelled by the solvent (mm) Observations: Table 1: Lip-print observations (5) Sample Lip-print Sketches Table 2: Chromatography data (5) Sample Distance Solvent Travelled Observations Distance Sample Travelled Rf Calculations Analysis: (10) In a paragraph discuss the following: Analyze the evidence to determine which one, if any, wrote the note. Explain your reasoning. How reliable is this information in court? Explain. If the chromatography paper was doubled in size or the solvent brand was changed do you expect the same Rf values? Explain. What are some other substances that can be separated using this process? Research one other chromatography technique (thin-layer chromatography, gas chromatography or high-performance chromatography) and report how it can be used to analyze evidence in court.