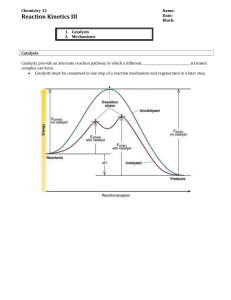
SI Exam III Review - Iowa State University
advertisement
Leader: Ryan Gale Course: Chem 178 Supplemental Instruction Instructor: Burnett/Raker Iowa State University Date: 4/14/13 1.) Which of the following parameters is not a kinetic property? SI Exam III Review a. b. c. d. Temperature Entropy Physical State Concentration 2.) Which of the following rates is representing an elementary reaction? a. b. c. d. A+B→C A→C A + 2B → 3C 2A + B → 3C Rate = [A]2[B] Rate = [C] Rate = [A][B]2 Rate = [C]3/([A]2[B]) 3.) Consider the following energy profile diagram. How many intermediates are formed, which energy hill is the rate limiting step, and is the reaction exothermic or endothermic, respectively? a. b. c. d. 3, 2nd, and endothermic 0, 1st, and endothermic 1, 2nd, and exothermic 1, 1st, and exothermic 4.) A reaction A + 2B → C obeys the following rate law: Rate = k[A]2[B]. If [B] is doubled and [A] is halved, how will the rate change? a. Rate will double b. Rate will half c. Rate will remain constant d. Rate will quadruple Supplemental Instruction 1060 Hixson-Lied Student Success Center 294-6624 www.si.iastate.edu 5.) For the following gas-phase reaction, what is the correct rate expression for the rate of disappearance of reactants and appearance of products for the reaction: 2 NO(g) + 2H2(g) → N2(g) + 2H2O(g) a. − b. − c. ∆[𝑁𝑂] ∆𝑡 =− 2∆[𝑁𝑂] ∆𝑡 ∆[𝑁𝑂] 2∆𝑡 = ∆[𝑁𝑂] ∆[𝐻2 ] ∆𝑡 =− ∆[𝐻2 ] 2∆𝑡 = 2∆[𝐻2 ] ∆𝑡 =− ∆[𝑁2 ] ∆𝑡 = ∆[𝑁2 ] ∆[𝑁2 ] ∆[𝐻2 ] 2∆𝑡 ∆[𝐻2 𝑂] = ∆𝑡 ∆𝑡 = =− ∆[𝑁 ] 2∆[𝐻2 𝑂] ∆𝑡 ∆[𝐻2 𝑂] 2∆𝑡 ∆[𝐻 𝑂] 2 = ∆𝑡2 = 2∆𝑡 6.) The reaction C2H5Br + OH → C2H5OH + Br is first order in hydroxide only. When [C2H5Br] = 0.005 M, [OH ] = 0.050 M, the reaction rate at 298 K is 0.0432 M/s. What is the half-life? d. − a. b. c. d. 2∆𝑡 =− 2∆𝑡 t1/2 = 0.080 s t1/2 = 0.80 s t1/2 = 0.60 s t1/2 = 0.0040 s 7.) For the reaction 2 NO2(g) → 2NO(g) + O2(g) the rate constant, k, for the reaction was found to be 0.023 M-1 s-1. What axis would yield a straight line for this reaction? a. [NO2] vs. time b. ln[NO2] vs. time c. 1/[NO2] vs. time d. exp[NO2] vs. time 8.) Indicate whether each statement is true or false. If it is false, correct it. a. If you measure the rate constant for a reaction at different temperatures, you can calculate the overall enthalpy change for the reaction b. Exothermic reactions are faster than endothermic reactions. c. If you double the temperature for a reaction, you cut the activation energy in half. 9.) What is the molecularity of the reaction : CH3COOH(aq) + OH (aq) → H2O(l) + CH3COO (aq) a. b. c. d. Unimolecular Bimolecular Termolecular This is not a mechanism, so the molecularity = 0 10.) The reaction 2 NO(g) + Cl2(g) → 2 NOCl(g) is carried out in a closed vessel. If the partial pressure of NO is decreasing at the rate of 56 torr/min, what is the rate of change of pressure of Cl2(g) in the vessel? a. b. c. d. 112 torr/min -28 torr/min 56 torr/min 28 torr/min 11.) In the lab, you have studied the gas-phase oxidation of HBr by O2: 4 HBr(g) + O2(g) → 2 H2O(g) + Br2(g) You find the reaction is first order in each of the 2 reactants. The following mechanism is proposed. Which step is the limiting step and what is the intermediate? HBr(g) + O2(g) → HOOBr(g) HOOBr(g) + HBr(g) → 2 HOBr(g) a. b. c. d. 1st step and HBr 2nd step and HOOBr 1st step and HOBr 1st step and HOOBr 12.) Based on the activation energies and energy changes, which of the following reactions would be fastest and which would be slowest? (i) Ea = 45 kJ/mol; ΔE = -25 kJ/mol (ii) Ea = 35 kJ/mol; ΔE = -10 kJ/mol (iii) Ea = 55 kJ/mol; ΔE = 10 kJ/mol a. i, ii b. i, iii c. ii, iii d. ii, i 13.) Predict the sign of the entropy change for: 3 C2H2(g) → C6H6(g) a. ΔS > 0 b. ΔS < 0 c. ΔS = 0 d. ΔS cannot be determined without thermodynamic data. 14.) For the process CO2(s) → CO2(g) a. b. c. d. 15.) ΔH > 0, ΔS < 0 ΔH < 0, ΔS < 0 ΔH < 0, ΔS > 0 ΔH > 0, ΔS > 0 Calculate the change in standard entropy for the reaction: Be(OH)2(s) → BeO(s) + H2O(g) S° 50.21, 13.77, 188.83 (J/molK) a. b. c. d. -152.39 J/K 152 J/K 166.16 J/K -102.18 J/K 16.) For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. Calculate ΔG° for this reaction at 298 K. a. b. c. d. 17.) -60.9 kJ -9.92 kJ 25,400 kJ -25,500 kJ Calculate the boiling point of elemental bromine, Br2(l) Br2(l) → Br2(g) ΔH°f = 30.71 kJ/mol; SBr2(g) = 245.3 J/molK; SBr2(l) = 152.3 J/molK a. b. c. d. 70 K 270 K 330 K 340 K 18.) Consider the gas-phase reaction between nitric acid and bromine at 273 °C: 2 NO(g) + Br2(g) → 2 NOBr(g) The following data for the initial rate of appearance of NOBr were obtained: a. Determine the rate law b. Calculate the rate constant c. What is the rate of disappearance of Br2 when [NO] = 0.075 M [Br2] = 0.25 M 19.) The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O: 2 NO(g) → N2O2(g) N2O2(g) + H2(g) → N2O(g) + H2O(g) a. Show that the elementary reactions of the proposed mechanism add to provide a balanced chemical reaction. b. Write a rate law for each elementary reaction in the mechanism. c. Identify any intermediates in the mechanism. d. The observed rate law is rate = k[NO]2[H2]. If the proposed mechanism is correct, what can we conclude about the relative speeds of the first and second reactions? 20.) A certain constant-pressure reaction is nonspontaneous at 45 °C. The entropy change for the reaction is 72 J/K. What can you conclude about the sign and magnitude of ΔH? 21.) The Kb = 4.4*10-4 for methylamine (CH3NH2) at 25 °C. a. Write the chemical equation for the equilibrium that corresponds to Kb. b. By using the value of Kb, calculate ΔG° for the equilibrium in part a. c. What is the value of ΔG at equilibrium? d. What is the value of ΔG when [H+] = 6.7 * 10-9 M, [CH3NH3+] = 2.4*10-3 M, and [CH3NH2] = 0.098 M? Formulas: