Notes
advertisement

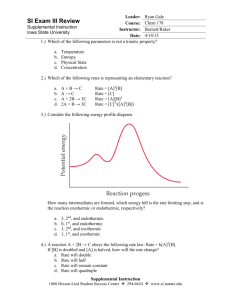
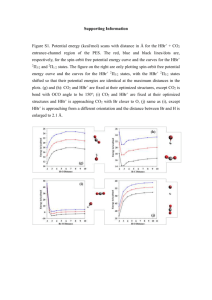
Chemistry 12 Reaction Kinetics III Name: Date: Block: 1. Catalysts 2. Mechanisms Catalysts Catalysts provide an alternate reaction pathway in which a different, ______________________________ activated complex can form. Catalysts must be consumed in one step of a reaction mechanism and regenerated in a later step. Mechanisms Reaction pathways are called _____________________. A reaction mechanism is a series of steps that may be added together to give an overall chemical reaction. Cannot be determined by just looking at overall reaction. Deduced through much study and research (up to years) You will not be asked to come up with mechanism from scratch. Some mechanisms are known, however, many are yet to be discovered. Example (known mechanism) 4 HBr + O2 → 2 H2O + 2 Br2 Mechanism (determined from lots of research) step 1: HBr + O2 → HOOBr (found to be slow) step 2: HBr + HOOBr → 2 HOBr (fast) step 3: HOBr + HBr → H2O + Br2 (very fast) Each step is called an ________________________ ___________________. An __________________________ is a species that is formed in one step and consumed in a subsequent step and does not appear in the overall reaction. List the intermediates in the reaction above: Rate determining step (RDS) - the _________________ step in the mechanism. The only way to speed up an overall reaction is to speed up the RDS (eg. by increasing. the concentration of a reactant in the RDS) Speeding up a fast step (not RDS) will have no effect on the overall rate. (eg. adding HOOBr or HOBr has no effect) To determining overall reaction, cancel stuff, which is identical on both sides - add up what’s left. 1.) A + 2X → AX2 2.) AX2 + X → AX + X2 3.) AX + A → A2 + X _____________________________________________________________________ Overall reaction: Intermediates: The following reaction occurs in a 3-step mechanism. Determine the 3rd step of the mechanism: 2A4+ + B+ → 2A3+ + B3+ step 1: A4+ + C2+ → C3+ + A3+ step 2: A4+ + C3+ → C4+ + A3+ step 3: ? Consider the following reaction for the formation of HCl in the presence of light. Determine the 2nd step of the mechanism. Cl2 + CHCl3 → HCl + CCl4 The following is the proposed reaction mechanism: Step 1: Cl2 → Cl + Cl Step 2: Step 3: Cl + CCl3 → CCl4 Returning back to… 4 HBr + O2 → 2 H2O + 2 Br2 step 1: step 2: step 3: HBr + O2 → HOOBr (found to be slow) HBr + HOOBr → 2 HOBr (fast) HOBr + HBr → H2O + Br2 (very fast) PE diagram for the reaction mechanism AC (step 1) AC (step 2) AC (step 3) HOOBr PE HOBr HBr + O2 STEP 1 STEP 2 STEP 3 H2O + Br2 REACTION PROCEEDS Notes: Each “bump” is a step The higher the bump, (greater Ea) the slower the step The highest bump (from the reactants level) is for the RDS AC’s at top of bumps, intermediates in middle “valleys”, products in the final “valley” The Ea for the forward overall reaction is vertical distance from reactants to top of highest bump. Given the following Potential Energy Diagram for a reaction mechanism: PE (KJ) 80 70 60 50 Reaction Proceeds 1. This mechanism has _____________ steps. Number each step. 2. Ea for overall reaction = ___________________kJ 3. Step ___________is the RDS 4. Step ___________ is the fastest step. 5. The overall reaction is _________ thermic. 6. = ______________ kJ 7. for reverse rx. = ______________kJ 8. Ea (reverse rx.) = ______________ kJ 9. RDS for reverse reaction is step _____________ Draw a Potential Energy Diagram for a reaction mechanism with 2 steps. The first step is fast and the second step is slow. The overall reaction is exothermic. With labeled arrows show the overall Activation Energy (Ea) and the for the forward reaction. PE Reaction Proceeds 1. In the following reaction mechanisms, identify: i. The catalyst ii. The reaction intermediates iii. The overall reaction a) CH2=CH2 + H+ CH3-CH2+ CH3-CH2+ + H2O CH2-CH2-OH + H+ b) A + B C C+DE+F E+BD+F 2. “All catalyzed reaction mechanisms have more than one step.” Why must this statement be true? 3. Supposed a catalyzed reaction is occurring in a reaction container. If the catalyst were removed would the reaction stop completely? Explain your answer. 4. Consider the following reaction mechanism: X + Y Z (very fast) Z + Y P (very fast) P + Y Q (slow) Suppose there were a catalyst that would work on step 1 and another catalyst that would work on step 3. Which catalyst would be ineffective in increasing the overall reaction? Why? Hebden Workbook Pg. 36 #62, 63