12.
advertisement
Chem 241-2012 Exam 3 Sample Questions Answers
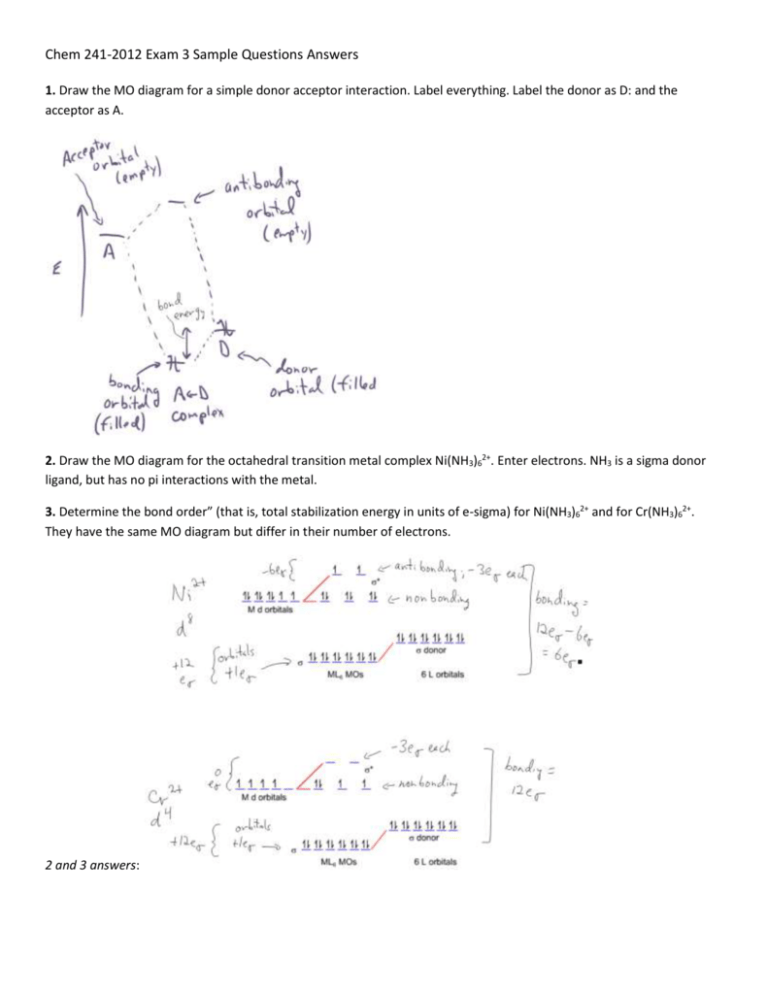
1. Draw the MO diagram for a simple donor acceptor interaction. Label everything. Label the donor as D: and the
acceptor as A.
2. Draw the MO diagram for the octahedral transition metal complex Ni(NH3)62+. Enter electrons. NH3 is a sigma donor
ligand, but has no pi interactions with the metal.
3. Determine the bond order” (that is, total stabilization energy in units of e-sigma) for Ni(NH3)62+ and for Cr(NH3)62+.
They have the same MO diagram but differ in their number of electrons.
2 and 3 answers:
4. For which d-electron configurations are different low- and high-spin cases possible in octahedral complexes?
d4, d5, d6, d7
5. Draw the MO diagrams for square planar and for tetrahedral 4-coordinate complexes. Determine the bond order for
both with each possible d-electron configuration (0 to 10 d electrons).
Which structure is preferred in each case?
When there is a tie in terms of bonding, which will prevail?
6. What type of transition leads to Ni(NH3)62+ having a green color?
Again, NH3 is a sigma-only ligand. If NH3 is replaced by oxalate ion (C2O42-) which is a pi-donor ligand, will the
absorption shift to higher or lower energy?
If NH3 is replaced by CO, which is a pi acceptor ligand, in which direction will the absorption shift?
7. Delete this question: We did not get to it. Which compound is more stable in terms of the ligands not falling off:
Ni(NH3)62+ or Ni(en)32+? Remember that “en” stands for ethylene diamine, which is a bidentate ligand that has similar
bond strength to the metal as NH3.
12. Considering only d-d transitions, which of the following cases will be colorless? Circle all that would be.
Zn2+
Cu2+
Cu+
low spin Mn2+
high spin Mn2+ high spin Fe3+
Sc3+
16. This lovely molecule is Co(NH3)3Cl2Br.
1. Is this isomer: cis, trans, mer, or fac.
2. Is this particular isomer chiral? SKIP THIS, but
answer is NO
3. How many other isomers can be formed with this
formula? Sketch them.
4a. What are the major structural differences between silicates and silicones? Draw structures to show what
you mean.
Silicates are composed of a Si-O backbone. Each Si atom is bonded to 4 O atoms. O atoms are bonded to either
one or two Si atoms. When an O atom is bonded to a single Si atom, that O atom is "terminal" and has three
lone pairs and a negative charge. When an O atom is bonded to two Si atoms it forms a Si-O-Si bridge.
Silicones can also contain Si-O-Si bridges but do NOT have terminal O atoms. The Si still has 4 bonds; any bonds
not to O atoms are to "alkyl" groups-- that is, groups like -CH3, -CH2CH3.
4b. There are three principal reagents used to make silicones. For each, indicate how much you would use to
make a liquid silicone with a relatively high boiling point.
Put Reagent formulas
down this column
circle one of these for each reagent
Use a lot
Use a little
Use none
Use a lot
Use a little
Use none
Use a lot
Use a little
Use none
5a. What’s a zeolite?
A zeolite is a 3-dimensional cage structure composed of a silica or silicate framework.
5b. What happens to a zeolite’s structure when Al atoms replace some Si atoms?
When an aluminum atom takes the place of a Si atom, the number of electrons in the structure stays the same,
but the number or protons decreases by 1 for each Al atom (because Al is atomic number 13, Si 14). Therefore,
each Al atom adds a negative charge to the structures. That means a cation needs to be introduced to match
that negative charge. It also means the Al-substituted zeolite will attract water.
14. What reaction or reactions lead to the initial formation of caves? That is, what makes a hole in the ground?
What reaction/reactions lead to the formation of, well, formations inside caves?
Cave formation occurs by two reactions: If there is acid in the water trickling into the CaCO3 rockbed, then the reaction is:
H+(aq) + CaCO3(s) Ca2+(aq) + HCO3-(aq)
{there are few variations on this theme}
If the water is passing through soil, it will be high in CO2. Then high CO2 concentration shifts equilibrium A toward H2CO3.
This H2CO3 reacts very favorably with CaCO3 to dissolve it to form HCO3-.(equilibrium B shifts right)
When the cave opens to the outside air, the reverse occurs. CO2 pressure decreases; this shifts equilibrium A away from
H2CO3. The decrease in H2CO3 shifts equilibrium B to the left- causing HCO3- to reform CaCO3(s).
15. What will result from a cave that forms, breaks the surface of the earth, and then a lot of time goes by? Lots of time.
What will you find when you go there?
I’m not answering this.
12. Consider the phase diagram for carbon and answer the associated questions. Note that this diagram has pressure
on the X-axis and temperature on the Y-axis.
a. Which point represents normal
conditions?
A
b. If you move graphite from A to D,
will it form diamond?
YES
c. What does “metastable” mean?
Thermodynamically unstable, but
kinetically stable. A metastable
species would be more stable if it
reacted but the reaction is
infinitely slow.











