Chapter 1-3 Review
advertisement

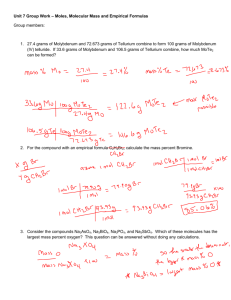
Tina Akinyi Chem 177 Pre-Exam Review – 9/10/15 __________________________________________________________________________ 1. a. The elemental analysis of methanol is 37.5 %C, 12.6 %H, and 49.9 %O. What is the empirical formula of methanol? b. What is the empirical formula of a compound that contains 10.4% C, 27.8% S, and 61.7% Cl by mass? c. What is the empirical formula of a compound that contains 32.79 % Na, 13.02% Al, and 54.19% F? 2. Which of the following will form a covalent bond? a. Nitrogen and oxygen b. Calcium and sulfur c. Zinc and chlorine d. Potassium and bromine 3. Give the following answer to the correct number of significant figures: a. [(12.67 + 19.2)] x 3.99] 1.367 b. How many significant figures are in: 2.060 x 10 -3 , 20600, and 0.0020600 ? 4. Rubidium has two naturally occurring isotopes, 85Rb (relative mass 84.9118 amu) and 87 Rb (relative mass 86.9092 amu). If rubidium has an average atomic mass of 85.47 amu, what is the abundance of each isotope (in percent)? 5. Give the formula for the following compounds: a. Lithium sulfate b. Beryllium phosphate c. Dinitrogen monoxide d. Ferric iodide e. Calcium acetate 6. Name the following compounds: a. FeSO4 b. K2S c. P2O5 d. Cl2O3 e. Cu(OH)2 7. Which of the following lengths is the largest? a. 6.7 m b. 8.9 x 10-4 km c. 7.3 x 1010 nm d. 5.4 x 103 mm 8. Calculate the following quantities: a. Mass, in grams, of 0.3322 moles of sucrose (C12H22O11) b. Moles of Zn(NO3)2 in 143.50 grams of this substance c. Number of molecules 1.0 x 10 -6 moles CH3CH2OH d. Number of N atoms in 0.410 mol NH3 9. A chemist set up a synthesis of phosphorus trichloride by mixing 18.8 g of white phosphorus (P4) with 59.4 g of chlorine gas and obtained 72.4 g of liquid phosphorus trichloride. a. Calculate the mass (in g) of phosphorus trichloride that can be made from the reactants. b. Calculate the percentage yield of the product. 10. In the following list, which is not an example of a chemical reaction? a. Dissolution of a penny in nitric acid b. A burning candle c. The condensation of water vapor d. The formation of polyethylene from ethylene 11. Identify element X. a. 64 29 X b. 108 47 X c. d. 200 39 80 X 19 X 12. Balance the equation: ____ Cu + ____ HNO3 → ____ Cu(NO3)2 + ____ NO + ____ H2O 13. How many molecules are in 3 moles of Cl2? 14. For the balanced equation shown below, what would be the limiting reagent if 76.0 grams of CH2Cl2 were reacted with 69.8 grams of O2? 2 CH2Cl2 + 3 O2 → 2 CO2 + 2 H2O + 2 Cl2 If 32.5 grams of CO2 are produced, what is the percent yield of the reaction? 15. Consider the following reaction: NH4NO3 + Na3PO4 → (NH4)PO4 + NaNO3 Which reactant is limiting, assuming we started with 30.0 grams of ammonium nitrate and 50.0 grams of sodium phosphate. What is the mass of each product that can be formed? What mass of the excess reactant(s) is left over? 16. What is the molecular formula of each of the following compounds? a. Empirical formula HCO2, molar mass= 90.0 g/mol b. Empirical formula C2H4O, molar mass=88 g/mol 17. You are running a series of experiments to determine the amount of carbon in a 10g of a liquid. The accepted value of carbon is 5g. Label each of the following as having a GOOD or BAD level of precision and accuracy a. 4.0g, 1.9 g, 9.9 g, 8.3 g b. 3.3 g, 3.2 g, 3.0 g, 3.2 g c. 5.1 g, 5.0 g, 4.9 g. 5.2 g 18. Draw propane, butane, pentane, and 2-propanol. Write the empirical formula of each compound. 19. Automobile batteries contain sulfuric acid, which is commonly referred to as battery acid. Calculate the number of grams of sulfuric acid in 1.00 gallon of battery acid if the solution has a density of 1.28 g/ml and is 38.1% sulfuric acid by mass. (1 Gallon= 3.78541 L)