Question 1: Find the molecular formula if a compound is composed
advertisement
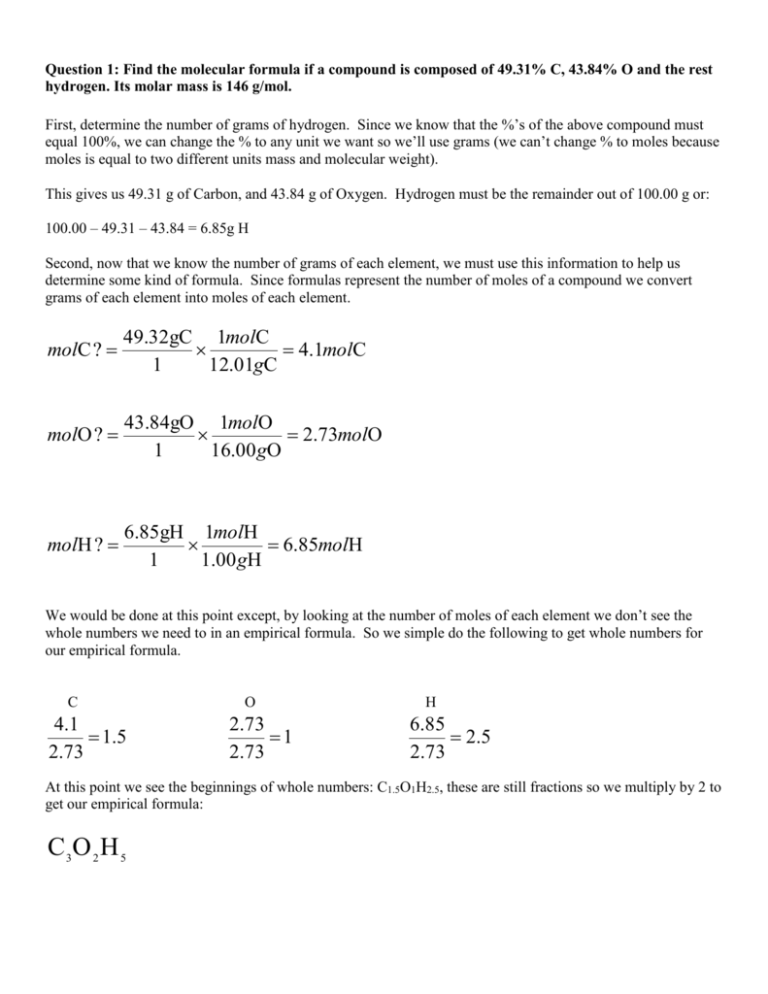
Question 1: Find the molecular formula if a compound is composed of 49.31% C, 43.84% O and the rest hydrogen. Its molar mass is 146 g/mol. First, determine the number of grams of hydrogen. Since we know that the %’s of the above compound must equal 100%, we can change the % to any unit we want so we’ll use grams (we can’t change % to moles because moles is equal to two different units mass and molecular weight). This gives us 49.31 g of Carbon, and 43.84 g of Oxygen. Hydrogen must be the remainder out of 100.00 g or: 100.00 – 49.31 – 43.84 = 6.85g H Second, now that we know the number of grams of each element, we must use this information to help us determine some kind of formula. Since formulas represent the number of moles of a compound we convert grams of each element into moles of each element. molC ? 49.32gC 1molC 4.1molC 1 12.01gC molO ? 43.84gO 1molO 2.73molO 1 16.00 gO molH ? 6.85gH 1molH 6.85molH 1 1.00 gH We would be done at this point except, by looking at the number of moles of each element we don’t see the whole numbers we need to in an empirical formula. So we simple do the following to get whole numbers for our empirical formula. C 4.1 1.5 2.73 O 2.73 1 2.73 H 6.85 2 .5 2.73 At this point we see the beginnings of whole numbers: C1.5O1H2.5, these are still fractions so we multiply by 2 to get our empirical formula: C3O 2 H 5 Calculating the molecular weight of the compound we get 73 g/mol. Since the molecular formula is 146 g/mol divide the 73 into 146: 146 2 73 We use this number to multiply our empirical formula to get our molecular formula: C 6 O 4 H10 Question 2: A 35.0 mg sample contains only C, H & N. Decomposition produced 33.5 mg carbon dioxide and 41.1 mg of water. What is the empirical formula? This question is in many way similar to the above. We have to go through one extra step…. at the very beginning…..to find the number of grams of C, H, & N; this is what we did in the last problem. From our lecture notes it said that we have to realize that to get the number of grams of C, H, & N we need to look at the mass of CO2 and H2O produced….because…we can find the mass of Carbon from CO2, and the mass of Hydrogen from H2O. Then, just like in the previous problem we get the mass of Nitrogen by subtracting the mass of C and H from our 35.00 mg sample. gC ? 33.5mgCO 2 1molCO 2 1molC 12.01gC 9.13mgC 1 44.01gCO 2 1molCO 2 1molC Note: I don’t convert mg to g…because I want mg at the end, and if you check…all of the units cancel except mgC. Same thing in the next step. gH ? 41.1gH 2 O 1molH 2 O 2molH 1.00 gH 4.56 gH 1 18.0 gH 2 O 1molH 2 O 1molH Now, to get the mass of Nitrogen subtract the masses of C and H from the original mass of the compound. 35.0 – 9.13 – 4.56 = 21.3 g N Having he grams of each compound….this should sound familiar….we start towards our empirical formula, which is based on moles, so we need to covert the masses of our elements to moles of elements. molC ? 9.13gC 1molC .76molC 1 12.01gC molH ? 4.56gH 1molH 4.56molH 1 1.00 gH molN ? 21.3gN 1molN 1.52molN 1 14.00 gN (Repeat of above since it is the exact same procedure) We would be done at this point except, by looking at the number of moles of each element we don’t see the whole numbers we need to in an empirical formula. So we simple do the following to get whole numbers for our empirical formula. C .76 1 .76 N 1.52 2 .76 At this point we whole numbers, our empirical formula: C1 H 6 N 2 H 4.56 2 .76