Chapter 18 Test 1. The nucleus of an atom
advertisement

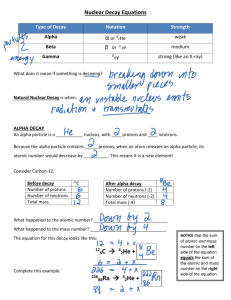
Chapter 18 Test 1. The nucleus of an atomA accounts for almost all of the mass of an atom B contains both protons and electrons C is negatively charged 2. Which of the following groups contains members with similar chemical reactivity? A Li, Be, C B C, N, O C Be, Mg, Sr 3. The table lists properties of some elements. Which element would you expect to be in group 18 of the periodic table? A carbon B neon C chlorine Chapter 18 Test 4. According to the periodic table, which element most readily accepts electrons? A* Fluorine B Arsenic C Nitrogen 5. An unidentified element has many of the same physical and chemical properties as magnesium and strontium but has a lower atomic mass than either of these elements. What is the most likely identity of this element? A Na B Ca C* Be 6. What type of nuclear decay is represented in this illustration? A beta decay B alpha decay C gamma radiation 7. Elements in Group 16 of the periodic table usually — A solidify at room temperature B gain electrons when bonding C act like metals Chapter 18 Test 8. The picture shows a model of the element — A fluorine B oxygen C* beryllium 9. The elements of which of these groups on the periodic table are most resistant to forming compounds? A Group 18 B Group 1 C Group 14 10. Which of the following represents an isotope of Carbon-14? A 8 6 B C 8 14 C C 14 6 C Chapter 18 Test 11. According to the graph, what is the approximate half-life of carbon-14? A 5.7 years B* 5,700years C 23,000 years 12. According to the periodic table, which of these elements will form an ion with a –2 charge? A S B F C Mg 13. Which of the following elements has the smallest atomic radius? A Sulfur B Aluminum C Chlorine 14. How many protons are in an atom of Cobalt-60? A 60 B 27 C 33 Chapter 18 Test 15. List the first three types of radioactive decay by the isotope below? 133 56 131 54 131 53 0 1 129 51 4 2 B Beta, Alpha, Beta C Alpha, Beta, Alpha 16. Most metallic elements have all of these properties EXCEPT — A a high melting point being B a good conductor of electricity C being easily crumbled into pieces 17. Which particle is located in the cloud region of the atom? A Electron B Nucleus C Neutron 18. Which of the following is the correct notation for an ion of chlorine? A 129 52 0 1 Ba Xe He I e Sb He Te e A Alpha, Beta, Gamma 4 2 Cl B Cl- C 35 17 Cl Chapter 18 Test 19. The table shows the atomic radii of some elements in Periods 1 through 4 of the periodic table. Which inference can be made from this information? A* Atomic radii decrease from left to right. B Atomic radii increase from bottom to top. C Atomic radii decrease from right to left. 20. Which of the following statements is correct for A contains more protons than neutrons B contains more electrons than protons C contains more neutrons than electrons ? Chapter 18 Test Use the graph below to answer questions 21-22 21. The graph shows the amount of carbon-14 in tissue over time. According to the graph, if a bone contains 1/8 the amount of carbon-14 that it did originally, its approximate age is — A 17,100 years B 22,800 years C 11,400 years 22. What type of nuclear decay is taking place in the graph? A Alpha B Beta C Gamma Chapter 18 Test 23. Elements found in which shaded area of this periodic table undergo the fewest chemical reactions? AS B T C Q 24. According to this information, which solid has an atomic mass greater than 200? A Rb B Cs C Th 25. Dot diagrams are used to represent ______. A the structure of the nucleus B atomic number C outer electrons