Introduction and the Cell
advertisement

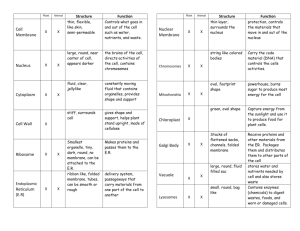
Lectures 2014 – 2015 Physiology and the concept of homeostasis Physiology is the study of the functions of the animal body. In other words, the mechanisms by which the various organs and tissues carry out their specific activities are considered . The goal of physiology is to explain the physical and chemical factors that are responsible for the origin, development, and progression of life. The body to function optimally, conditions within the body, referred to as the internal environment, must be very carefully regulated. Therefore, many important variables, such as body temperature, blood pressure, blood glucose, oxygen and carbon dioxide content of the blood, as well as electrolyte balance, are actively maintained within narrow physiological limits. Cells as the Living Units of the Body The basic living unit of the body is the cell. Each organ is an aggregate of many different cells held together by intercellular supporting structures. Each type of cell is specially adapted to perform one or a few particular functions. For instance, the red blood cells, transport oxygen from the lungs to the tissues. Body fluids About 60 percent of the adult animal body is fluid, mainly a water solution of ions and other substances. Although most of this fluid is inside the cells and is called intracellular fluid, about one third is in the spaces outside the cells and is called extracellular fluid. This extracellular fluid is in constant motion throughout the body. It is transported rapidly in the circulating blood and then mixed between the blood and the tissue fluids by diffusion through the capillary walls. In the extracellular fluid are the ions and nutrients needed by the cells to maintain cell life. Thus, all cells live in essentially the same environment—the extracellular fluid. For this reason, the extracellular fluid is also called the internal environment of the body, or the milieu intérieur, a term introduced more than 100 years ago by the great 19th-century French physiologist Claude Bernard. Homeostasis The term homeostasis is used by physiologists to mean maintenance of nearly constant conditions in the internal environment. It is important because the cells and tissues of the body will survive and function efficiently only when these internal conditions are properly maintained. The body is constantly faced with a changing external environment as well as with events and activities occurring within it that may alter the balance of important variables. For example, most metabolic reactions within cells consume oxygen and glucose. These substances must then be replaced. In addition, these reactions produce metabolic wastes including carbon dioxide and urea, which must then be eliminated. Therefore, it is more accurate to say that the internal environment is in a dynamic steady state — one that is constantly changing, but in which optimal conditions are physiologically maintained. All of the organ systems in the body, except the reproductive system, contribute to the maintenance of homeostasis . For example, the gastrointestinal tract digests foods to provide nutrients to the body. The respiratory system obtains oxygen and eliminates carbon dioxide. The circulatory system transports all of these materials and others from one part of the body to another. The renal system eliminates wastes and plays a role in regulating blood volume and blood pressure. Two regulatory systems in the body influence the activity of all the other organ systems so that homeostasis is ultimately maintained: 1-Nervous system : The sensory division of the peripheral nervous system is sensitive to changes in the internal and external environment. The information gathered by this component is transmitted to the CNS where it is processed, integrated, and interpreted. The CNS then determines the appropriate response to this input. This response is carried out by the transmission of nerve impulses in the motor division of the peripheral nervous system the effector tissues. 2-Endocrine system : The other regulatory system in the body contributing to the maintenance of homeostasis is the endocrine system, which carries out its effects by secreting hormones. These hormones are transported in the blood to the specific tissues upon which they exert their effects. In general, the nervous system primarily regulates muscular activity and glandular secretion and the endocrine system primarily regulates metabolic activity in the body’s cells. However, these two systems may work together in the regulation of many organs, as well as influence each other’s activity. Negative feedback Most of the body’s compensatory homeostatic mechanisms function by way of negative feedback. This is a response that causes the level of a variable to change in a direction opposite to that of the initial change. For example, when blood pressure increases, the arterial baroreceptors are stimulated and an increased number of nerve impulses are transmitted to the CNS through afferent pathways. The region of the brain regulating the cardiovascular system responds to this sensory input by altering efferent nerve activity to the heart. The result is a decrease in heart rate and therefore a decrease in blood pressure back to its baseline value. In general, when a physiological variable becomes too high or too low, a control system elicits a negative feedback response consisting of one or a series of changes that returns the variable to within its normal physiological range. These compensatory mechanisms operating via negative feedback allow the body to maintain homeostasis effectively. Positive Feedback Most control systems of the body operate by negative feedback rather than positive feedback . Positive feedback is better known as a “vicious cycle,” but in some instances, the body uses positive feedback to its advantage. Blood clotting is an example of a valuable use of positive feedback. When a blood vessel is ruptured and a clot begins to form, multiple enzymes called clotting factors are activated within the clot itself. Some of these enzymes act on other unactivated enzymes of the immediately adjacent blood, thus causing more blood clotting. This process continues until the hole in the vessel is plugged and bleeding no longer occurs. Childbirth is another instance in which positive feedback plays a valuable role. When uterine contractions become strong enough for the baby’s head to begin pushing through the cervix, stretch of the cervix sends signals through the uterine muscle back to the body of the uterus, causing even more powerful contractions. Thus, the uterine contractions stretch the cervix and the cervical stretch causes stronger contractions. When this process becomes powerful enough, the baby is born. If it is not powerful enough, the contractions usually die out and a few days pass before they begin again. The Cell Each one of the cells in an animal is a living structure that can survive for months or many years. Its two major parts are the nucleus and the cytoplasm. The nucleus is separated from the cytoplasm by a nuclear membrane, and the cytoplasm is separated from the surrounding fluids by a cell membrane, also called the plasma membrane. The different substances that make up the cell are collectively called protoplasm. Protoplasm is composed mainly of five basic substances: water, electrolytes, proteins, lipids, and carbohydrates. Plasma membrane Each cell is surrounded by a plasma membrane that separates the cytoplasmic contents of the cell, or the intracellular fluid, from the fluid outside the cell, the extracellular fluid. An important homeostatic function of this plasma membrane is to serve as a permeability barrier that insulates or protects the cytoplasm from immediate changes in the surrounding environment. Furthermore, it allows the cell to maintain a cytoplasmic composition very different from that of the extracellular fluid; the functions of neurons and muscle cells depend on this difference. The plasma membrane also contains many enzymes and other components such as antigens and receptors that allow cells to interact with other cells, neurotransmitters, blood-borne substances such as hormones, and various other chemical substances, such as drugs. Structure and function of plasma membrane The major components of the plasma membrane include: 1-Phospholipids 2- Cholesterol 3-Proteins 4- Carbohydrates The basic structure of the plasma membrane is formed by phospholipids , which are one of the more abundant of the membrane components. Phospholipids are amphipathic molecules that have polar (water-soluble) and nonpolar (water-insoluble) regions. They are composed of a phosphorylated glycerol backbone, which forms a hydrophilic polar head group and a nonpolar region containing two hydrophobic fatty acid chains. In an aqueous environment such as the body, these molecules are arranged in a formation referred to as the lipid bilayer consisting of two layers of phospholipids. The polar region of the molecule is oriented toward the outer surface of the membrane where it can interact with water; the nonpolar, hydrophobic fatty acids are in the center of the membrane away from the water. The functional significance of this lipid bilayer is that it creates a semipermeable barrier . Lipophilic, or nonwater-soluble, substances can readily cross the membrane by simply passing through its lipid core. Important examples of these substances include gases, such as oxygen and carbon dioxide, and fatty acid molecules, which are used to form energy within muscle cells. Most hydrophilic, or water-soluble, substances are repelled by this hydrophobic interior and cannot simply diffuse through the membrane. Instead, these substances must cross the membrane using specialized transport mechanisms. Examples of lipidinsoluble substances that require such mechanisms include nutrient molecules, such as glucose and amino acids, and all species of ions (Na+ , Ca++ , H+ , Cl– , and HCO3). Therefore, the plasma membrane plays a very important role in determining the composition of the intracellular fluid by selectively permitting substances to move in and out of the cell. Another important aspect of the lipid bilayer is that the phospholipids are not held together by chemical bonds. This enables molecules to move about freely within the membrane, resulting in a structure that is not rigid in nature, but instead, very fluid and pliable. Also contributing to membrane fluidity is the presence of cholesterol. Cholesterol prevents the fatty acid chains from packing together and crystallizing, which would decrease membrane fluidity. Membrane fluidity is very important in terms of function in many cell types. For example, skeletal muscle activity involves shortening and lengthening of muscle fibers. Furthermore, as white blood cells leave the blood vessels and enter the tissue spaces to fight infection, they must squeeze through tiny pores in the wall of the capillary requiring significant deformation of the cell and its membrane. Finally, in all cells, many processes that transport substances across the plasma membrane require the embedded proteins to change their conformation and move about within the bilayer. In each case, in order for the cell membrane, or the entire cell, to change its shape, the membrane must be very fluid and flexible. Proteins are also associated with the lipid bilayer and essentially float within it. Intrinsic proteins are embedded within and span the membrane, and extrinsic proteins are found on the internal or external surface of the membrane . These proteins provide a variety of important cellular functions by forming the following structures: • Channels • Carrier molecules • Enzymes • Chemical receptors • Antigens Some proteins may form channels through the cell membrane that allow small, watersoluble substances such as ions to enter or leave the cell. Other proteins may serve as carrier molecules that selectively transport larger water-soluble molecules, such as glucose or cellular products, across the membrane. Regulators of specific chemical reactions, enzymes are extrinsic proteins found on the internal (e.g., adenylate cyclase) or external (e.g., acetylcholinesterase) surfaces of the membrane. Chemical receptors are found on the outer surface of the cell membrane and selectively bind with various endogenous molecules as well as with drugs. Through receptor activation, many substances unable to enter the cell and cause a direct intracellular effect may indirectly influence intracellular activity without actually crossing the membrane. Other proteins found on the external surface of the plasma membrane are antigens . These molecules serve as cell “markers” that allow the body’s immune system to distinguish between its own cells and foreign cells or organisms such as bacteria and viruses. The plasma membrane contains a small amount of carbohydrate (2 to 10% of the mass of the membrane) on the outer surface. This carbohydrate is found attached to most of the protein molecules, forming glycoproteins, and to some of the phospholipid molecules (<10%), forming glycolipids. Consequently, the external surface of the cell has a carbohydrate coat, or glycocalyx. These carbohydrate moieties have several important functions, including: • Repelling negatively charged substances: many of the carbohydrates are negatively charged, creating an overall negative charge on the surface of the cell that repels negatively charged extracellular molecules. • Cell-to-cell attachment: the glycocalyx of one cell may attach to the glycocalyx of another cell, which causes the cells to become attached. • Receptors: carbohydrates may also serve as specific membrane receptors for extracellular substances such as hormones. • Immune reactions: carbohydrates play a role in the ability of cells to distinguish between “self” cells and foreign cells. Membrane transport The lipid bilayer arrangement of the plasma membrane renders it selectively permeable. Uncharged or nonpolar molecules, such as oxygen, carbon dioxide, and fatty acids, are lipid soluble and may permeate through the membrane quite readily. Charged or polar molecules, such as glucose, proteins, and ions, are water soluble and impermeable, unable to cross the membrane unassisted. These substances require protein channels or carrier molecules to enter or leave the cell. Passive diffusion through the membrane Molecules and ions are in constant motion and the velocity of their motion is proportional to their temperature. This passive movement of molecules and ions from one place to another is referred to as diffusion . When a molecule is unevenly distributed across a permeable membrane with a higher concentration on one side and a lower concentration on the opposite side, there is said to be a concentration gradient or a concentration difference. Although all of the molecules are in motion, the tendency is for a greater number of molecules to move from the area of high concentration toward the area of low concentration. This uneven movement of molecules is referred to as net diffusion. The net diffusion of molecules continues until the concentrations of the substance on both sides of the membrane are equal and the subsequent movement of molecules through the membrane is in a dynamic equilibrium . In other words, the number of molecules moving in one direction across the membrane is equal to the number of molecules moving in the opposite direction. At this point, although the diffusion of molecules continues, no further net diffusion takes place. The movement of ions, in particular, depends not only on a concentration gradient but also on an electrical gradient . Positively charged ions (cations) are attracted to a negatively charged area and negatively charged ions (anions) are attracted to a positively charged area. Ions of a similar charge tend to repel each other and oppose diffusion. Diffusion of Ions Through Protein Channels integral membrane proteins can span the lipid bilayer. Some of these proteins form channels that allow ions (such as Na+, K+, Cl–, and Ca2+ ) to diffuse across the membrane. Ion channels can exist in an open or closed state , and changes in a membrane’s permeability to ions can occur rapidly as these channels open or close. The process of opening and closing ion channels is known as channel gating, like the opening and closing of a gate in a fence. Three factors can alter the channel protein conformations, producing changes in how long or how often a channel opens. First, the binding of specific molecules to channel proteins produce change in the shape of the channel protein. Such channels are termed ligand-gated channels, and the ligands that influence them are often chemical messengers. Second, changes in the membrane potential can cause movement of the charged regions on a channel protein, altering its shape—these are voltagegated channels. Third, physically deforming (stretching) the membrane may affect the conformation of some channel proteins— these are mechanically-gated channels. A particular type of ion may pass through several different types of channels. For example, a membrane may contain ligand-gated K+ channels, voltage-gated K+ channels, and mechanically-gated K+ channels. Moreover, the same membrane may have several types of voltage-gated K+ channels, each responding to a different range of membrane voltage, or several types of ligand-gated K+ channels, each responding to a different chemical messenger. Facilitated Diffusion Facilitated diffusion uses a transporter to move solute “downhill” from a higher to a lower concentration across a membrane. Neither diffusion nor facilitated diffusion is coupled to energy (ATP) derived from metabolism. Thus, they are incapable of moving solute from a lower to a higher concentration across a membrane. Among the most important facilitated-diffusion systems in the body are those that move glucose across plasma membranes. Without such glucose transporters, cells would be virtually impermeable to glucose, a relatively large, polar molecule. Osmosis Osmosis is the net movement of water through a semipermeable membrane down its own concentration gradient from an area of high water concentration to an area of low water concentration. In other words, water moves toward an area of higher solute concentration. The osmotic pressure of a solution is the pressure or force by which water is drawn into the solution through a semipermeable membrane. The osmolarity of the extracellular fluid is normally in the range of 285–300 mOsm. If a RBC is placed in a solution of 300 mOsm, they will neither swell nor shrink because the water concentrations in the intra- and extracellular fluid are the same, and the solutes cannot leave or enter. Such solutions are said to be isotonic. By contrast, hypotonic solutions have a solute concentration lower than that found in cells, and therefore water moves by osmosis into the cells, causing them to swell. Similarly, solutions containing greater than 300 mOsm of solutes (hypertonic solutions) cause cells to shrink as water diffuses out of the cell into the fluid with the lower water concentration. Active transport With active transport , energy is expended to move a substance against its concentration gradient from an area of low concentration to an area of high concentration. This process is used to accumulate a substance on one side of the plasma membrane or the other. The most common example of active transport is the sodium–potassium pump that involves the activity of Na+ –K+ ATPase, an intrinsic membrane protein. For each ATP molecule hydrolyzed by Na+ –K+ ATPase, this pump moves three Na+ ions out of the cell and two K+ ions into it. Two means of coupling an energy flow to transporters are known: (1) the direct use of ATP in primary active transport, and (2) the use of an electrochemical gradient across a membrane to drive the process in secondary active transport. One of the best studied examples of primary active transport is the movement of sodium and potassium ions across plasma membranes by the Na+/K+-ATPase pump. Secondary active transport is distinguished from primary active transport by its use of an electrochemical gradient across a plasma membrane as its energy source, rather than phosphorylation of a transport molecule by ATP. In secondary active transport, the movement of an ion down its electrochemical gradient is coupled to the transport of another molecule, such as a nutrient like glucose or an amino acid. The movement of the actively transported solute can be either in the same direction as sodium, in which case it is known as cotransport, or opposite the direction of sodium movement, which is called countertransport . The terms symport and antiport are also used to refer to the processes of cotransport and countertransport, respectively.