Biodiesel from Coffee Grounds
advertisement

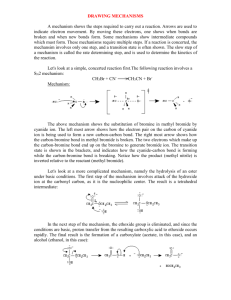
Biodiesel from Coffee Grounds Timikeyi Egbuson 12/05/2013 Chemistry 213. 101 Michael Banales Synthetic FFR 2 1|Egbuson Introduction Transesterification of vegetable oils dates back to the mid 1800s where glycerin was often distilled for the manufacture of soap. 17th century chemists, E.Duffy and J.Patrick, are credited for the genesis of transesterification using vegetable oils before the first diesel engine became operational.1 While cellular organisms produce vast amounts of organic compounds, pigments such as indigo and alizarin are isolated from crude aqueous extracts. The general isolation strategies of natural products include solvent extraction, fractionation, chromatography and purification.2 Owing to the gradual scarcity of existing oil and gas reserves, there is the need to find different energy sources that replace depleting fossil fuels. Biodiesel is considered the cutting edge alternative and has prominently become the viable substitute for compressionignition engines due to its non-toxicity, renewability and biodegradability. Biodiesel is the monoalkyl ester of triglyceride oil and is produced by chemically combining triglyceride with an alcohol in acidic or basic catalyst. Unlike fossil fuels, biodiesels tackle environmental degradation due to its ecofriendly nature, liquid state, and portability. They also do not emit carbon monoxide or other harmful gases when combusted. The ability of its botanical sources (via photosynthesis) to ingest the carbon dioxide, which it releases, explains why it does not contribute to global warming. Global concerns have erupted because this fuel is primarily obtained from soybean oil or other cereals and producing fuel from food is not so rational considering the rise in world population. As a result, other sources are being sought for biodiesel production. These include Galicia marine algae, fungi, sewage sludges, invasive species and use of ultrasonic reactors.3 Transesterification, otherwise called alcoholysis, involves the transformation of esters via displacement of an alkoxy moiety. Generally, it occurs in equilibrium and a catalyst usually modifies and regulates equilibria rates. Excess alcohol is usually used to achieve high ester yield. Transesterification is prominent in industrial processes 2|Egbuson involving synthesis of polyethylene terephthalate and not just constrained to laboratory scale.4 Catalyst type, reaction temperature, alcohol/oil molar ratio and lipid content are important factors that affect transesterification. Coffee grounds have high lipid content, which is about 15% by dry weight. 5 Scheme 1. Mechanism of base-catalyzed transesterification of triglyceride 5 Methanol reacts with potassium carbonate, producing an alkoxide and potassium bicarbonate. The resulting alkoxide undergoes nucleophilic acyl substitution on triglyceride, giving rise to a tetrahedral intermediate, which undergoes cleavage forming fatty acid methyl esters (biodiesel) and glycerol. Triglyceride oil was isolated and biodiesel was synthesized via base-catalyzed transesterification. PH paper was used to test for a neutral pH while extraction was employed in the workup based on the principle of aqueous and organic layers. Percent yield and recovery calculations were determined while infrared spectroscopy and gas chromatography data confirmed purity, product formation and composition. 3|Egbuson Experimental Isolation. Brewed wet coffee grounds were dried for 2 days at 50°C. Dried coffee grounds (50g) and hexanes (150mL) were combined and refluxed for 1.5 hours with continuous stirring. Upon cooling, amber viscous triglyceride oil was removed via vacuum filtration. (2.457g, 4.914%) IR (ATR) υmax (cm-1) 2922.1, 2853.4, 1742.9, 1459.6, 1158.3, 1093.6. Base-catalyzed Transesterification. Triglyceride oil (2.457g, 2.79mmol), methanol (1.45mL) and potassium carbonate (0.184g) were combined and refluxed for 30 minutes with stirring. Workup. Acetic acid (1M, 1mL), hexanes (10mL) and 90% methanol/water mixture (5mL) were added to the reaction mixture and pipette mixed. Liquid-liquid extraction yielded biodiesel as a dark brown, viscous liquid. (2.229g, 88.9%); IR (ATR) υmax (cm-1) 2922.0, 2853.4, 1459.2, 1372, 1160.0; GC-MS (phenyl methylpolysiloxane, 125 oC to 275 oC at 10 oC per min) RT 11.18 min (270.24 m/z), 11.60 min (256.01 m/z), 12.84 min (294.06 m/z), 12.88 min (296.05 m/z), 13.10 min (298.78 m/z), 13.25 min (281.44 m/z), 14.86 min (332.02 m/z). Discussion Base-catalyzed transesterification is relatively faster than the acid-catalyzed reaction.5 This explains the use of potassium carbonate in the transesterification of glyceride to synthesize biodiesel. A 4.9% recovery of triglyceride oil was obtained from coffee grounds while a 88% yield of biodiesel was derived based on the isolated triglyceride oil. Based on coffee type used, a moderate 5.9% yield for biodiesel was successfully extracted in comparison to that of literature (10-15%).5 4|Egbuson Gas chromatography-mass spectroscopy data was analyzed to show the content composition of biodiesel. At retention time 11.18 min, methyl palmitate had a parent peak at 270.24 m/z with relative abundance one tenth of the base peak. The base peak ion at m/z = 73.86 undergoes McLafferty rearrangement loosing the methyl ester which is fragmented between the α and β substituted carbons while the ion at m/z = 86.89 is fragmented between C4 – C5 also loosing methyl ester and a hydrogen atom. At retention time 11.6 min, palmatic acid’s parent peak corresponds to 256.01 m/z. An ion with m/z = 56.97 is fragmented between C3 and C4 loosing a methylene diol and three hydrogen atoms via McLafferty rearrangement while the ion at 72.85 m/z also represents fragmentation of the carboxylic acid between C3 and C4 loosing a propanoate. At retention time 12.84 min, methyl linoleate’s molecular ion occurs at 294.06 m/z. Both ions at m/z = 67.02 and 80.90 represent hydrocarbon fragments with general formula [CnH2n-3] loosing dialkenes and a hydrogen atom. At retention time 12.88 min, methyl oleate’s parent peak is observed at m/z = 296.05. The peak at m/z = 73.90 represents the rearranged McLafferty methyl ester fragment while the peak at 86.87 m/z represents fragmented hydrocarbon ions with general formula [CH3OCO(CH2)n]. At retention time 13.10 min, methyl stearate’s molecular ion occurs at m/z = 298.78. The ion present at m/z = 73.96 corresponds to the McLafferty rearranged methyl ester fragmented between the α and β carbons while the ion at m/z = 87.01 represents loss of methyl ester and a hydrogen atom fragmented between C3-C4 respectively. At retention time 13.25 min, linoleic acid’s molecular ion at m/z = 281.44 represents water loss from carboxyl group while both ions at m/z = 67.24 and 80.93 represent hydrocarbon fragments with general formula [CnH2n-3] due to loss of alkenes and a hydrogen atom. At retention time 14.86 min, methyl arachisate’s parent peak occurs at m/z = 332.03. The ion at m/z = 74 represents loss of the McLafferty rearranged methyl ester fragmented between C2 5|Egbuson –C3 while the ion at m/z = 87.10 represents the C4-C5 fragmented methyl ester and hydrogen atom. In most cases, a similar fragmentation pattern was observed for the methyl esters and fatty acids. As shown by the spectra data, the synthesized biodiesel primarily comprises of methyl palmitate (20.89 weight%), palmatic acid (12.05 weight%), methyl linoeate (23.26 weight%), methyl oleate (7.87 weight%), methyl stearate (5.29 weight%), linoleic acid (27.16 weight%) and methyl archiste (3.44 weight%). This matches the content and similar composition trends as reported in literature.3 The presence of these fatty acid mono alkyl esters and the observed viscous nature of the product formed validate the formation of biodiesel. The formed carboxylic acids occurred as a result of saponification, the hydrolysis of the fatty acid esters in excess heat. Infrared spectra data was also analyzed for the triglyceride oil and biodiesel fuel, hence proving its formation. The strong, intense peaks observed between 2853.4 – 2922.1 cm-1 represent the three terminal alkyl carbon-hydrogen stretches in triglyceride, while the medium to strong peak observed at 1742.9 cm-1 corresponds to the carbonyl group present in the aliphatic oil. The moderate scissor bend observed at 1459.6 cm-1 represents the methylene group present in triglyceride and the moderately intense peaks between 1093.6 – 1158.3 cm-1 are characterized by the alkyl esters in the viscous oil. On the other hand, the conspicuous and strong peaks between 2853.4 – 2922.0 cm-1 represent the terminal alkyl groups in biodiesel. Furthermore, the strong peak at 1742.6 cm-1 corresponds to the carbonyl functional group present in the fuel. Meanwhile, the moderate umbrella bend observed between 1372.7 – 1459.2 cm-1 represents the methyl hydrocarbon within the biodiesel structure. The moderate peak observed at 1160.0 cm-1 is characterized by the alkyl esters of the biodiesel. In general, the peaks present in the biodiesel spectra closely resemble that of the triglyceride spectra due to extremely similar structures. However, the distinct umbrella bend of the methyl moiety at 1372.7 cm-1 present in the biodiesel 6|Egbuson spectra, and not in the triglyceride spectra, confirms the successful formation of the desired biodiesel fuel. Also, the presence of fewer peaks in the fingerprint region of the biodiesel spectra in comparison to that of the triglyceride further confirms formation of the fuel product. This is as a result of the biodiesel being monoalkylated, unlike the more complex tri-alkylated triglyceride oil. In future conditions, the transesterification process of triglycerde oil should be done via acid catalysis to obtain significantly higher yield. Also, extraction of triglyceride oil from coffee grounds should be done with a variety of solvents (polar and non polar) to obtain optimal percent recovery. The importance of biodiesel cannot be overemphasized in our contemprorary society as it proffers to mankind a solution to energy crisis due to its renewability, portability and clean nature. References 1) 2) 3) 4) 5) McNeill, G.P.; Shimizu, S.; Yamane, T. J. Am. Oil Chem. Soc. 1991, 68, 1. Bajwa, U.; Bains, G.S. J. Food. Sci. Technol. 1987, 24, 81. Kondamudi N.; Mohapatra S.K; Misra M. J. Agric Food Chem. 2008, 56, 11757 – 11760. Otera, J. Chem. Rev., 1993, 93, 1449. Braz. J. Chem Soc., 1998, 199 – 210, 1998 7|Egbuson