Abstract (PDF)
advertisement

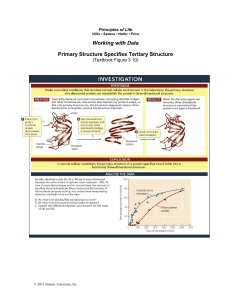
Characterizing the glycoforms of RNase 1, and, Developing fluorogenic indicators of cytosolic entry Robert G. Presler, Jr. Prof. Ronald T. Raines Dept. of Biochemsitry, Integrated Program in Biochemistry (IPiB) The secreted, RNA-degrading protein bovine ribonuclease A (RNase A) is perhaps the most studied enzyme of the 20th century. RNase A was the first enzyme to have its chemical mechanism understood, it was among the first protein structures to be solved, and its stability is legendary (classically, RNase A purification relies upon its ability to survive brutal acidification and heatdenaturation). Nevertheless, despite half a century of scientific intimacy with this 14-kDa bovine protein, much of the biology and biochemistry of the native human homolog (RNase 1) remains unclear. Rigorous characterization of native human RNase 1 has proven to be challenging. The current inability to efficiently separate and study individual glycoforms of native RNase 1 greatly limits scientific inquiry. To illustrate this point, bovine RNase A can only be decorated with a single glycan (to yield “RNase B”); human RNase 1, however, can potentiate as many as seven individual glycoforms. Thus, whereas RNases A and B can be easily separated, RNase 1 can only be subdivided into four fractions: (I) mono-, (II) di-, (III) tri-, and (IV) unglycosylated. Illustrated aside, fractions (I) and (II) are actually a mixture of individual glycoforms. Herein, a strategy is described to produce all seven RNase 1 glycoforms, thus enabling rigorous biochemical and cell biological analyses. Cell biology has been revolutionized by the ability to detect otherwise invisible macromolecules by using small-molecule fluorophores. For example, esterase-labile latent fluorophores have enabled the kinetic resolution of RNase A endosomal entry. A key step in RNase A internalization, cytosolic entry, has yet to be sufficiently resolved. In this research effort, a strategy is described for the generation of cytosol-sensing latent fluorophores.