TITRATION OF ACIDS AND BASES Inquiry LAB 1112
advertisement

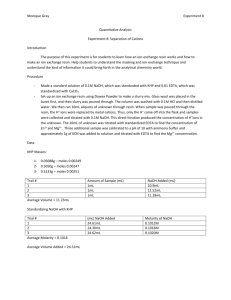
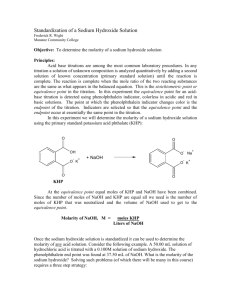
TITRATION OF ACIDS AND BASES LAB Source: Nelson and Kemp You will develop a procedure for standardizing a solution of NaOH of unknown molarity and then use this solution to determine the molarity of a solution of HCl. Background: Review the text, sections 4.5 and 4.6, paying attention to the section on titrations. Refer to your class notes and problem solving for section 4.6 as well (the PowerPoint for 4.6 is online on the Honors Chemistry main page under the Titration of Acids and Bases Lab section). Present your procedure to the teacher for approval prior to conducting the lab. Review lab 1 for the calculation of standard deviation. Pre Lab Questions 1. Define standardization as it relates to this lab. 2. Define titration. 3. Define molarity. 4. What are equivalence points and end points and how do they differ? 5. What is parallax error and how might it be a problem with the equipment in this lab? How can it be avoided? 6. Why is it necessary to make the solutions with distilled water that has been previously boiled? 7. What is the molarity of a solution that contains 3.78g of H2C2O4·2H2O in 200. mL of solution? 8. If 50.0 mL of Na OH is required to react completely with 0.62g of KHP, what is the molarity of the solution? Specifics: Part 1: Preparing the NaOH Solution Make 1 L of a 0.1000 M solution of NaOH starting with the solid reagent. Use boiled distilled water to make the solution. Prepare your data table. Part 2: Standardization of the Base Standardize the NaOH solution against a primary standard, KHP (potassium hydrogen phthalate, molar mass 204.2 g/mol). Determine how much KHP is needed to react with between 10.00 and 15.00 mL of the NaOH solution you prepared. You will place the KHP into a flask with 100 mL of boiled distilled water and 2 to 3 drops of phenolphthalein indicator. Perform 3 trials and perform a statistical analysis to determine the molarity of the base to 4 places after the decimal +/- SD. Prepare your data table. Part 3: Determining the Volume of a Drop Since it is possible that the endpoint of the titration may be reached with less than a full drop of NaOH, you have to determine the volume of a drop released from your buret. Use water to do this as you clean your buret. HINT: A calculation must be performed to determine the volume of a drop. Prepare your data table. Part 4: Determine the Molarity of an HCl Solution Report the molarity of the NaOH to the teacher to receive a sample of HCl of an appropriate molarity. Add between 5 and 10 mL of the acid to a flask with 100 mL of boiled distilled water and 2 to 3 drops of the phenolphthalein indicator. Perform 3 trials and perform a statistical analysis to determine the molarity of the acid to 4 places after the decimal +/- SD. Determine your Percent Error for the molarity of the HCl. Prepare your data table. Part 5: Lab Report Complete all parts of the lab report as usual. There is no graph. Post Lab Questions 1. A solution of malonic acid, H2C3H2O4 , was standardized by titration with 0.100M NaOH solution. If 20.76mL of the NaOH solution were required to neutralize completely 13.15mL of the malonic acid solution, what is the molarity of the malonic acid solution? 2. Sodium carbonate is a reagent that may be used to standardize acids in the same way that you have used KHP in this experiment. In such a standardization it was found that a 0.498g sample of sodium carbonate required 23.5mL of sulfuric acid to reach the end point for the reaction. What is the molarity of the sulfuric acid?