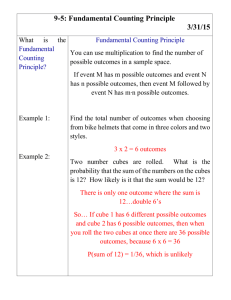
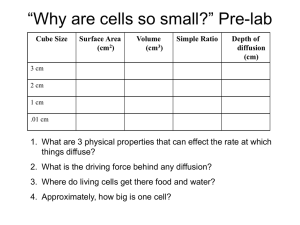
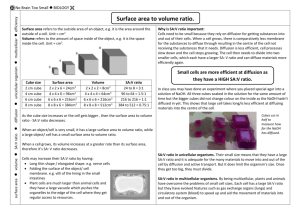
DENSITY PRACTICE
advertisement

DENSITY CUBE LAB REMEMBER: D=m/v (Density=Mass/Volume) 1. Using the ruler; find the length, width, and height of each of the nine cubes. Use these dimensions to calculate volume. *Remember V=lxwxh. 2. Using the triple beam balance, find the mass of each cube. 3. Using the formula D=m/v, calculate the density of each cube. 4. Match the density you calculated with the densities listed on the chart to determine the identity of each cube. 5. List the cube numbers in order from least dense to most dense and write a conclusion. DENSITY CUBE LAB REMEMBER: D=m/v (Density=Mass/Volume) 1. Using the ruler; find the length, width, and height of each of the nine cubes. Use these dimensions to calculate volume. *Remember V=lxwxh. 2. Using the triple beam balance, find the mass of each cube. 3. Using the formula D=m/v, calculate the density of each cube. 4. Match the density you calculated with the densities listed on the chart to determine the identity of each cube. 5. List the cube numbers in order from least dense to most dense and write a conclusion. Last Updated 6/11/2012 Page 1 of 4 Density Cube Lab Cube # Volume (in cm3) lxwxh Mass (in g) Density formula (D=m/v) Density (in g/cm3) Float or sink in water? Identity of cube 1 2 3 4 5 6 7 8 9 List the cube numbers in order from least dense to most dense: Last Updated 6/11/2012 Page 2 of 4 Conclusion: Answer the following questions in complete sentences in the form of a paragraph. What was the purpose of today’s lab? What properties did you use to find density? What formula did you use to calculate density? Why was the volume the same for all the cubes? If the all the cubes were the same size, why did they have different densities? Which cube number was the least dense and which one was the most dense? If the density of water is 1 g/cm3, which cubes would float? Conclusion: Answer the following questions in complete sentences in the form of a paragraph. What was the purpose of today’s lab? What properties did you use to find density? What formula did you use to calculate density? Why was the volume the same for all the cubes? If the all the cubes were the same size, why did they have different densities? Which cube number was the least dense and which one was the most dense? If the density of water is 1 g/cm3, which cubes would float? Last Updated 6/11/2012 Page 3 of 4 Teacher’s Note: Use the Constant Volume density cube set from Science Kit. It comes with a list of the types of cubes and the density of each one. Make a copy of that chart to give to each lab group once they have finished calculating the densities for each of the 9 cubes. They will then use the chart to identify the cubes. Before the lab you will need to number the cubes 1-9 and make a key according to how you’ve numbered them. When students are calculating the density of each cube, instruct them to round their answers to the nearest hundredth. Last Updated 6/11/2012 Page 4 of 4