Module 3 SA to V Ratio PowerPoint
advertisement

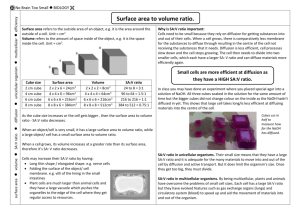
Introduction to Nanotechnology Module 3 Surface Area to Volume Ratio The “Big Ideas” of Nanoscale Science* Sense of Scale Surface area to volume ratio Density, force and pressure Surface tension Priority of forces at different size scales Material/Surface properties Understanding of these concepts requires an integration of the disciplines of math, biology, chemistry, physics and engineering © Deb Newberry 2008 Surface Area to Volume Ratio • This ratio is an important factor in understanding many of the straightforward, counter intuitive or unusual properties that can be observed at the nanoscale. • Surface Area to Volume ratio (SA/V) changes as the size of the material changes – it is not constant!!!! – Not convinced? Do the calculation for a die and a Rubics cube. • Also – if we keep the total volume of a material constant but divide that volume into smaller and smaller pieces – the SA does not increase in a linear fashion. Surface Area to Volume Ratio • The SA/V ratio also represents the percentage of the atoms that are on the surface of the material. • SA drives chemical, electrical and biological interactions and systems • V drives weight, cost, inertia, momentum and other factors • Both SA and V are dependent on a linear dimension (length) but SA goes as the square and V goes as the cube – this is “rub” • Ratios of this type are found in many equations – this is the first encounter to get familiar with this concept – it can be found in all aspects of nanoscience Surface area to volume Sugar Cubes Basic algebra Rules of exponents, Units conversion Surface area to volume Other shapes Excel optional Soap bubbles Pressure, force and density For a cube: V= a3 Surface area =6 a2 Notice the difference in powers of the linear dimension in the ratio of surface area to volume (SA/V) a a a Breaking the large cube into smaller cubes keeps the Total volume the same but Increase the total surface area Impacts: •Cell sizes •Surface tension •Nanotex pants Ref: NanoInk SA/V represents the percentage of atoms on the surface of an entity Let’s assume we have a cube that is 1 cm 3 The SA will then be 6 cm 2 Assume each atom is 1 nm 3 in size and takes up an area on the surface of 1 nm 2 How many atoms in the cube? How many atoms on the surface? What is the percentage? Now break the larger cube into cubes 1 mm on a side Percentage of total atoms that are on the surface Percentage of atoms on the surface for each smaller cube Surface area to volume ratio • Changes for an object as the size of that object changes • Impacts percentage of atoms on the surface that are available to participate in reactions • Changes non linearly as a large object is broken down into smaller objects • Introduces us to thinking about the dependence of different parameters on different powers of the linear dimension Start looking for…. • “Hidden” dimensional dependencies • At first glance pressure only appears to be dependent on the area aspect of the length dimension… • But upon closer inspection – see we have a volume dependence in the numerator…… • This happens many times in all of the traditional sciences. • This critical thing concept extends to other parameters (temperature, material properties etc.) References • Poole, Charles P., and Frank J. Owens. Introduction to Nanotechnology. Hoboken, NJ: J. Wiley, 2003.