Lecture 3. Water As An Environment A. Fundamental Characteristics
advertisement

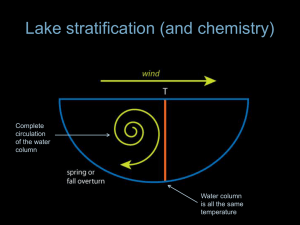
Lecture 3. Water As An Environment A. Fundamental Characteristics of Water Water, the environment of lakes, is an unfamiliar, alien environment for people. Water differs from air in being denser and in holding more heat and less oxygen per unit volume. Water exists in three phases (that is, physically distinct forms of matter): a solid phase – ice; a liquid phase – water; and a gaseous phase – water vapor. Like air, water is a fluid, having a low internal (e.g. crystalline) structure and thus distorting rather than breaking when they receive a force. By this definition, water and ice are both fluids; the solid form simply distorts more slowly. As on land, the fundamental physical characteristics of water constrain possibilities of life in lakes and streams. B. Water As A Substance Here, we address the small-scale physical and chemical characteristics of water which impact ecological structure and function. 1. Shape and Polarity The water molecule has two hydrogen atoms jutting out from one side of an oxygen atom. Because the two hydrogen atoms are on one side of the oxygen, the molecule is polar, with a negative pole on the side away from the oxygen atoms and a positive pole between the hydrogen atoms. The polar water molecule readily forms weak hydrogen bonds with other polar molecules, including water itself. Hydrogen bonds are the result of attraction between the negative pole of one molecule and the positive pole of another. The tendency of water to form hydrogen bonds has important consequences, e.g. viscosity, a density which changes with temperature, ability to act as a solvent and its role as a coolant. [T]: Dodson, Figure 2.1, p. 30 2. Viscosity Because water molecules tend to bond together, energy is needed to change their relative positions. This phenomenon is perceived by us as an internal ‘stickiness’ which is termed viscosity. The movement of particles (including small organisms) is significantly slowed in water. While we don’t sense this ‘stickiness’ viscosity is a major environmental constrain for small particles (<1 mm; e.g. clays and small organisms). [T]: Dodson, Figure 2.2, p. 31 [T]: Stokes’ Law The viscosity of water is inversely proportion to temperature, approximately doubling over the range of temperatures characteristic of north temperate lakes. [T]: Viscosity = f (temperature) This temperature dependency can impact lakes both seasonally and spatially. 3. Density Density refers to the weight of a substance per unit volume. One cubic centimeter (one milliliter) of water weighs one gram at 3.98 C. The density of a substance depends on its molecular composition and the distance between molecules, with more closely packed molecules having greater density. The temperature-density relationship for water is unusual in that the liquid is more dense that the solid. Thus, ice floats on liquid water. The density of water decrease as the liquid gets either warmer or colder than its temperature of maximum density, 3.98 C. [T] Dodson, Figure 2.5, p. 38. 4. Heat, Cooling and Evaporation Heat is a form of energy, measured in units such as calories. One calorie is defined as the amount of heat required to raise the temperature of water from 14.5 C to 15.5 C. Temperature is a sensation of hot or cold, but is related to heat and energy as adding heat to a substance generally (adding heat to impart a phase change doesn’t change temperature) increases the temperature. Expressed in another fashion, specific heat is the amount of heat per unit mass (e.g. calories/gram C) required to raise the temperature of a substance by 1 C. The specific heat of water is 1 calorie/gram °C, which is higher than any other common substance. As a result, water plays a very important role in temperature regulation. Lakes both warm and cool much more slowly than the ambient air temperature and large bodies of water, including the Great Lakes serve to moderate local and regional climate. Evaporation occurs when liquid molecules escape the water surface and enter the vapor phase. For each gram of water that evaporates, 540 calories are removed from the liquid water resulting in a cooling effect. Evaporation can play a significant role in the heat budget of lakes and surface cooling can help to mix surface waters as the cooler, denser waters sink. Applications: Density, Viscosity and Heating/Cooling (1) Density and Thermal Stratification The study of stratification is a study of layers. In limnology, those layers are study as a group, termed a profile, e.g. a vertical temperature profile. Thus, an examination of thermal stratification considers the formation of layers of differing temperature in an isothermal lake and the return of those stratified waters to an isothermal condition. If we begin our examination in early spring, we would find the lake to be isothermal, i.e. the temperature is essentially the same from top to bottom. [T] Dodson, Figure 2.9, p. 42. An isothermal lake mixes well over the entire depth of the water column and we say that it is completely-mixed or turning over. Shortly after ice-out, the water temperature would be ~4 C. The solar gain characteristic of spring warms the water and it may continue to circulate leading to a wellmixed water column with temperature of 5 C, 6 C, 7 C, etc. [T] Dodson, Figure 2.7, p. 40 On a still and warm day in spring, heat will collect near the surface of an isothermal lake and the floating, warmed surface waters will mix with themselves rather than the deeper, colder water below. [T] Dodson, Figure 2.6, p. 40 At this point we say that the lake is thermally stratified. During summer stratification, the lake possesses three distinct regions: a warm well-mixed surface layer, the epilimnion, an intermediate layer where temperature is in transition, the metalimnion and a cold, bottom layer, the hypolimnion. We define the metalimnion as the region where temperature is changing by at least 1 C per meter and the thermocline as the depth of maximum temperature change within the metalimnion. Summer winds tend to keep the epilimnion well mixed. The region of transition in temperature, i.e. the metalimnion, is maintained through density differences there which resist mixing. [T] Wetzel RTM However, shear at the boundary between the epilimnion and metalimnion tends to ‘erode’ the colder waters leading to a deepening of the epilimnion over the summer. [T] Dodson, Figure 2.7, p. 41 This deepening of the epilimnion tends to shrink the volume of the hypolimnion rather than warm it. The hypolimnion warms only slightly over the summer (1-2 C) and is less well mixed than the epilimnion because there is less physically energy input there. [T] Dodson, Figure 2.7, p. 42 In fall, cooler weather and shorter days begin to cool the surface waters. This cooler water sinks below the still warmer waters of the epilimnion until the epilimnion and the hypolimnion temperatures approach one another. At this point density differences are small and the lake mixes throughout the water column leading to isothermal conditions. This event is called fall turnover (or overturn). Continued cooling of the surface waters past 4 C leads to inverse or winter stratification with ice at the surface and the most dense (4 C) waters at the bottom. [T] Following ice-out in spring, the lake again warms to isothermal conditions, an event termed spring turnover (or overturn) and the annual process begins again. Continue with: Factors governing thermal stratification Brief discussion of importance to mixing (more later with chemistry) Other annual mixing patterns Then, (2) Viscosity: DCM, BNL (3) Heating/Cooling