Clinical Waste Transfer Notice
advertisement
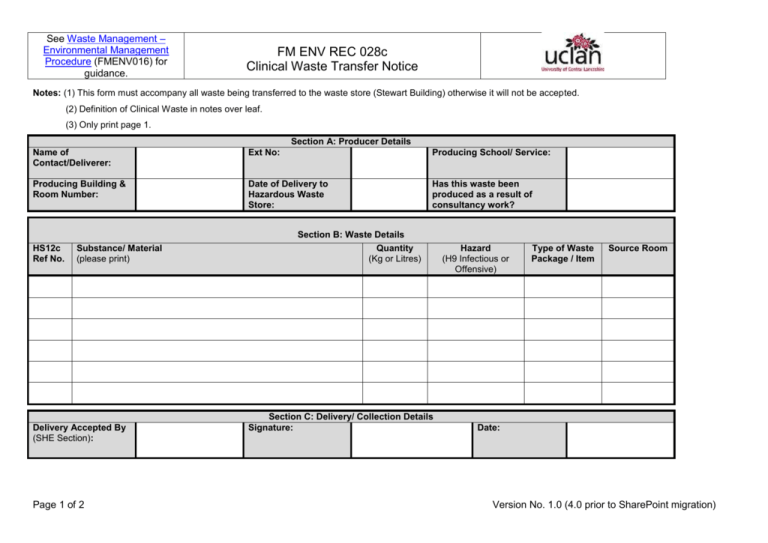
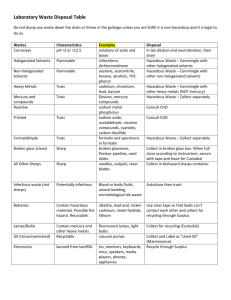
See Waste Management – Environmental Management Procedure (FMENV016) for guidance. FM ENV REC 028c Clinical Waste Transfer Notice Notes: (1) This form must accompany all waste being transferred to the waste store (Stewart Building) otherwise it will not be accepted. (2) Definition of Clinical Waste in notes over leaf. (3) Only print page 1. Section A: Producer Details Name of Contact/Deliverer: Ext No: Producing School/ Service: Producing Building & Room Number: Date of Delivery to Hazardous Waste Store: Has this waste been produced as a result of consultancy work? HS12c Ref No. Substance/ Material (please print) Delivery Accepted By (SHE Section): Page 1 of 2 Section B: Waste Details Quantity (Kg or Litres) Section C: Delivery/ Collection Details Signature: Hazard (H9 Infectious or Offensive) Type of Waste Package / Item Source Room Date: Version No. 1.0 (4.0 prior to SharePoint migration) See Waste Management – Environmental Management Procedure (FMENV016) for guidance. FM ENV REC 028c Clinical Waste Transfer Notice CLINICAL WASTE 1.1 Clinical Waste Is the waste harmful, irritant, toxic, corrosive, oxidising, carcinogenic, mutagenic, teratogenic, ecotoxic or senstitzing Does the waste meet the Clinical Waste definition given in 1.1 NO YES YES Does risk assessment, analysis or knowledge indicate that the waste is likely to contain a Microbial toxin? NO Refer to the Hazardous Waste Disposal Procedure Does the toxin(s) concentration render the waste Harmful or Toxic? Is the waste a culture or enrichment of a micro-organism reliably believed to cause disease in man or other living animal? Could "the waste cause infection to any person, (or other living organism), ‘coming into contact with it ?" YES And “any other waste arising from medical, nursing, dental, veterinary, pharmaceutical or similar practice, investigation, treatment, care, teaching or research, or the collection of blood for transfusion., being waste which may cause infection to any person coming in contact with it”. Producers of waste that has been treated (e.g. via autoclaving) and who do not want to deal with it as clinical waste under environmental legislation, need to be able to show to the Environment Agency that it is: is safe and non-infectious; and cannot be distinguished from other similar non-clinical wastes. NO Not Hazardous by H9 the waste may be Offensive Waste PageSection 2 of 2 5.4.3 Refer to the NonHazardous Waste Disposal Procedure YES Does risk assessment, analysis or knowledge indicate that the waste is likely to contain a Human / animal pathogen above naturally encountered levels ? NO NO The legal definition of clinical waste is: “any waste which consists wholly or partly of infectious human or animal tissue, blood or other bodily fluids, excretions, drugs or other pharmaceutical products, swabs or dressings, or syringes, needles or other sharp instruments, being waste which unless rendered safe may prove hazardous to any person coming into contact with it”. YES Waste is Hazardous by H9 Infectious and either H5 Harmful or H6 Toxic In these circumstances, the waste may be classed as non-clinical waste or offensive waste. The most effective kind of evidence is documentary proof that the waste has been suitably and successfully treated to ensure the removal of any hazard to anyone who may come into contact with it. Waste is Hazardous by H9 Infectious Version No. 1.0 (4.0 prior to SharePoint migration)