Heat Transfer Infrared Energy Definitions Radiation transfer of
advertisement

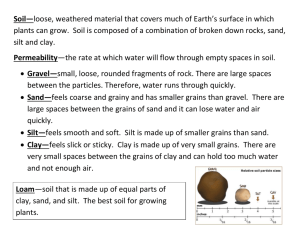
Heat Transfer Infrared Energy Definitions Radiation transfer of energy by way of electromagnetic waves Conduction transfer of heat from a warmer molecule to a cooler molecule Convection transfer of heat through a fluid (gas or liquid) from an area of higher temperature to a an area of lower temperature Thermal energy total energy of a substance's particles due to their movement or vibration Temperature a measure of the average energy of motion of particles of a substance image source: http://www.geography.hunter.cuny.edu/~tbw/wc.notes/2.heating.earth.surface/mechanisms_heat_transfer.htm A lamp with an incandescent bulb was used as a heat source. The lamp emits light and long-wave radiation called infrared. The beakers contain either sand, soil, gravel, or water. Each beaker had a lamp. A starting temperature was recorded for each material. The lamp was turned on for 10 minutes and then turned off for 10 minutes. Temperatures were recorded at the end of 10 minutes of heating and after 10 minutes of cooling. Changes in thermal energy were measured using an electronic temperature probe. White paper and black paper were included in the experiment to study the effect of color on heat transfer. Data Analysis Heat energy was transferred from the lamp to the sand by radiation. Heat spread through the sand by conduction. When the sand cooled, heat was transferred into the air by conduction. Air moves up from the sand by convection Heat transfer is the same as in sand. The gravel retains thermal energy because the gravel chunks are denser than sand. Heat transfer is the same as in sand. Soil is darker than sand and therefore absorbed more radiation. The soil particles were loose and released the heat energy. The soil was also dry. Heat transfer is the same as for sand, except that heat also circulates in water by convection. Water has a high heat capacity because of the shape of the water molecule. Heat is transferred to the paper from the lamp by radiation. Black paper will absorb most of the light and convert it to infrared. Therefore, black paper will be warmer than white paper. Heat is transferred to the paper from the lamp by radiation. White paper reflects most of the light energy and little is converted to infrared. Therefore, white paper remains cooler than black paper. Conclusions Light colored materials will absorb less light and infrared energy than dark colored materials and will be cooler by comparison. Dense materials will conduct heat more readily than less dense materials. If this is true, then blacktop should convert more solar energy into heat than light colored concrete even though both materials are dense. Soil although dark should be cooler than either blacktop or concrete. Areas near water may be cooler because of convection occurring over water due to a temperature difference between a body of water and land.