c 9 fraction basis of pyrocondensate stabilizers
advertisement

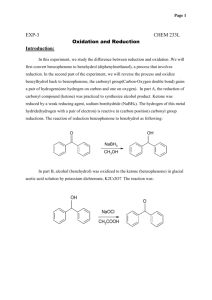
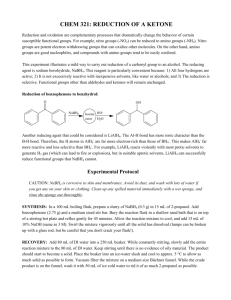
SYNTHESIS OF C8-C9 FRACTION BASIS OF PYROCONDENSATE STABILIZERS Ch.G. Rasulov, A.H.Azizov, E.A.Majidov, M.M.Chalishkan Azerbaijan NAS Institute of Petrochemical Processes named after Yu.G.Mamedaliyev e-mail: mcaliskan@qu.edu.az Pyrolysis of low-octane petrol has become widespread in the world. As a result, large quantities of hydrocarbons are released into the biosphere. Compounds which are produced from pyrolysis plants still have not found their applicable areas. In this work, it is mentioned that the fraction of 130-190°С liquid products of the pyrolysis of low-octane petrol and para-arylalkylphenol which is obtained by the catalytic arylalkylation of phenols are reacted with acetic acid and benzoyl chloride to obtain aceto- and benzophenone which are used as photo stabilizer in polystyrene. The liquid product of C8 – C9 fraction of pyrolysis (at 130-190°С) contains upto 36.6% of styrene. Beside styrene there are other kind of unsaturated hydrocarbons within the fraction of 130-190oC, such as: α-methylstyrene -6.15% ,vinyltoluene-7.02%, indene-4.85%. The fraction of 130-190°C has the following properties: Tb.=130-190°С, 20 𝑛𝐷 1.5156, 𝜌420 0.8583, molecular weight 120. Arylalkylation reactions were carried out using KU-23, phosphoric acidimpregnated zeolite-Y, seokar-2 and seokar-2M catalysts. The process has been accomplished by using batch and continuous devices. The desired product was separated by the rectification of obtained alkylate. The physical and chemical properties of para-arylalkylphenol have been determined as Tb.=160-180°С (at 10 mmHg.); 𝑛𝐷20 1.5730, 𝜌440 0.9825; molecular weight 200. By the acylation reaction of para-arylalkylphenol, accordingly aceto- and bezophenones have been synthesized. The synthesis of monoacylated products from the reaction between cyclophenols and organic acids in the presence of Lewis catalysts.The reaction is carried out in this method: The mixture of 16.5 grams (0.27mole) of glacial acetic acid and 16.5 grams (0.12 mol) of ZnCl 2 are added into a150 ml three-necked round bottom flask. The mixture is heated up to 90 0C and slowly 0.1 mol cyclophenol is added to the mixture .The reaction is carried out between 120-1600C for between 20 to 60 minutes . Then the mixture is washed with aqueous solution of HCl and at low pressure the mixture is distillated. At the end, the product is washed with ethyl alcohol so acetophenone is obtained The methods of benzoylation of cyclophenols : 0.1 mole of cyclophenol and KU-23 catalyst (catalyst amount – 10-20 % by mass on cyclophenol) are added into a100 ml three-necked round bottom flask.The mixture are heated up to 100 oC then 0.1 mole of benzoyl chloride is added dropwise to the mixture and heating is continued for 30 minutes.Then temperature increases between 140-160 oC and the reaction is carried out for 8-10 hours. After the reaction is stopped , let the reaction cool to 60 oC and filter the mixture to separate from catalyst . Add cancentrated NaOH solution to precipitate benzophenone and again filter it .Purify with column chromatography . So benzophenone is obtained. The chemical structure and the physical and chemical properties of the produced aceto- and bezophenones were determined. Aceto- and bezophenones were tested as photo-stabilizer in polystyrene. In contrast to the known stabilizers when aceto – and benzophenones as proposed after the tests results were added into styrene, it was determined that optical constant density of polystyrene samples was constant after photo-irradiation at 8 hours. REFERENCES : 1. Majidov E.A., İbrahimov H.J., Azizov A.H., Rasulov Ch.G. // Azerbaijan Oil Industry, 2013, №7-8, p.71-74. 2. Rasulov Ch.G., Azizov A.H., Azimova R.K., Abbasov S.İ. // Petro-chemistry, 2012, t.52, №5, p.1-5.