Flame Colours activity_student - vje-chem2011-test
advertisement

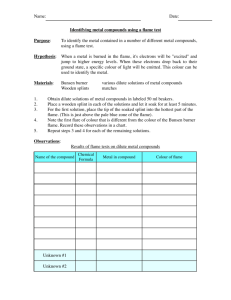
Flame Colours: How fireworks get their colour Name:______________________ Group / Partners:___________________________ Date:________________ Background Have you ever wondered how fireworks get their colour, or what makes neon signs red? We have the answer right in front of us when we are looking at the periodic table. Different elements when heated produce can emit light of a certain wavelength (colour). Some metals emit light within the visible spectrum. The colour we observe is specific for the metal that caused it and is a result of the excitation of the electrons in the metal atom. When the electrons are excited due to the extra energy provided by the flame they move to a higher energy level, when the electron relaxes back to its ground state the extra energy is emitted as light. In this experiment we will observe the colours imparted to the flame with the naked eye, and with a spectroscope. We will then use the information contained in our results identify the metal cation in an unknown compound. Safety: Ensure you have completed a risk assessment form, signed it and handed it to the teacher before commencing the experiment. ** DO NOT leave the flame in the watch glass for long periods this will cause the glass to crack and break. DO NOT attempt to lift the watch glass with your hand it will burn!. Be careful when wearing gloves around a Bunsen burner the last thing you want is to melt the latex onto your hands. Aim 1 Equipment: Equipment Heat resistant mat 100 mL Beaker Watch Glasses (1 for each metal salt) Spatula Bunsen Burner & matches Hand Held Spectroscope Chemicals 100mL of 2M HCl Sodium Carbonate, Potassium Carbonate, Calcium Carbonate, Strontium Carbonate, Barium Carbonate, Copper Carbonate, unknown compound. Method: 1. Place each compound to be tested on a watch glass (a small spatula full, about the size of a pea), when you are ready to conduct the test place the watch glass on the beaker. 2. Light the Bunsen burner ready to conduct the experiment. Check your partner is ready with the spectroscope. 3. Add a small amount of 2M HCl (a few mL) to the compound until it fizzes. 4. Using a blue flame hold the Bunsen burner over the fizzing compound. 5. Observe the colour produced and record it in the table below. 6. Your partner should use the spectroscope to observe the spectral colours, and record this as well. 7. Repeat for all of the carbonates and the unknown compound record your observations and the readings form the spectroscope in the table below. Results: Compound Tested Observed Colour Spectral Colours Observed 400 450 500 550 600 650 700nm Na2CO3 K2CO3 CaCO3 SrCO3 BaCO3 CuCO3 Unknown Visual Spectrum White Light UV 2 V B G Y O R Infra Red Questions: 1. Using your result identify the metal cation present in your unknown sample. 2. How can you be sure that the metal cation is responsible for the colour produced in the 3. 4. 5. 6. 7. flame and not the non-metal anion? What is the gas you observe being produced (fizzing) when the HCl is added to the metal carbonate? Explain how the metal cations produced the colours of light observed. Why do different metals give off different colours? Write the ground state electron configuration for sodium and a possible excited state. Why is examining the emission of light through the spectroscope superior to simply observing the change in the flame colour? When heated in a flame the sodium atoms in a sample of sodium carbonate are excited and light is emitted. Examination of the light with a spectroscope shows the following wavelengths to be present: 330.2, 330.3, 568.3, 568.8, 589.0 and 589.6 nm. Explain how these wavelengths are produced and what conclusions scientists such as Neils Bohr and Erwin Schroedinger came to about the structure of the atom after examining such wavelengths. 3