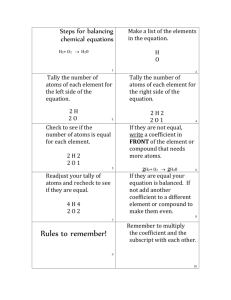
Chemical Formulas & Counting Atoms Practice!
advertisement
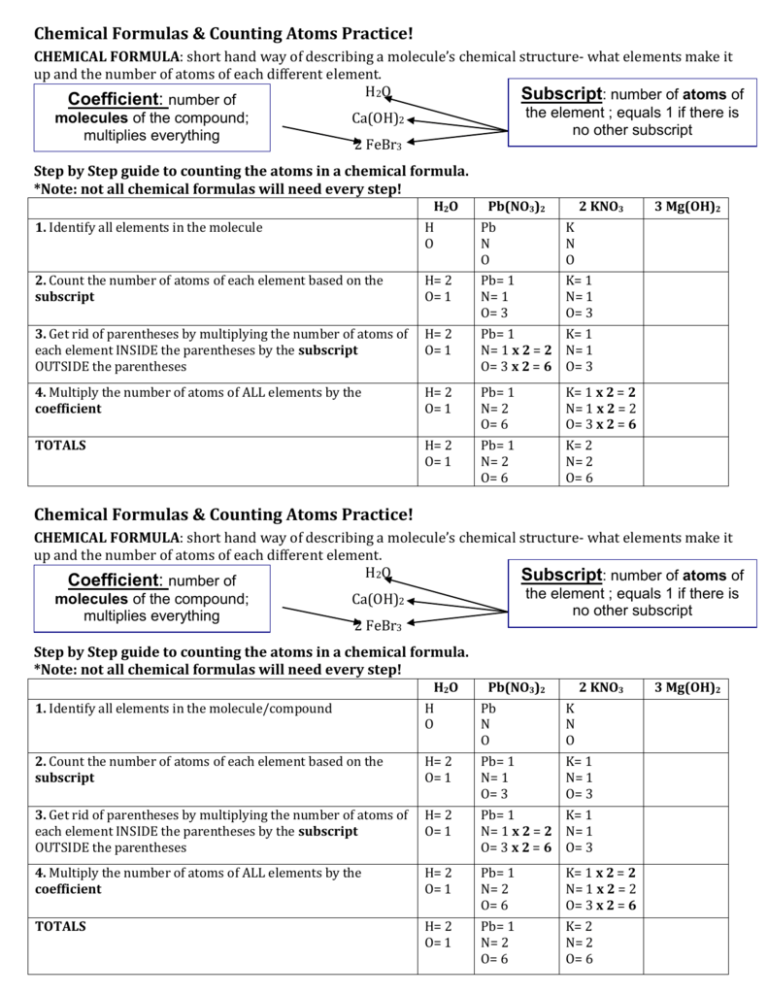
Chemical Formulas & Counting Atoms Practice! CHEMICAL FORMULA: short hand way of describing a molecule’s chemical structure- what elements make it up and the number of atoms of each different element. H2O Subscript: number of atoms of Coefficient: number of the element ; equals 1 if there is molecules of the compound; Ca(OH)2 no other subscript multiplies everything 2 FeBr3 Step by Step guide to counting the atoms in a chemical formula. *Note: not all chemical formulas will need every step! H2O Pb(NO3)2 2 KNO3 1. Identify all elements in the molecule H O Pb N O K N O 2. Count the number of atoms of each element based on the subscript H= 2 O= 1 Pb= 1 N= 1 O= 3 K= 1 N= 1 O= 3 3. Get rid of parentheses by multiplying the number of atoms of each element INSIDE the parentheses by the subscript OUTSIDE the parentheses H= 2 O= 1 Pb= 1 K= 1 N= 1 x 2 = 2 N= 1 O= 3 x 2 = 6 O= 3 4. Multiply the number of atoms of ALL elements by the coefficient H= 2 O= 1 Pb= 1 N= 2 O= 6 K= 1 x 2 = 2 N= 1 x 2 = 2 O= 3 x 2 = 6 TOTALS H= 2 O= 1 Pb= 1 N= 2 O= 6 K= 2 N= 2 O= 6 3 Mg(OH)2 Chemical Formulas & Counting Atoms Practice! CHEMICAL FORMULA: short hand way of describing a molecule’s chemical structure- what elements make it up and the number of atoms of each different element. H2O Subscript: number of atoms of Coefficient: number of the element ; equals 1 if there is molecules of the compound; Ca(OH)2 no other subscript multiplies everything 2 FeBr3 Step by Step guide to counting the atoms in a chemical formula. *Note: not all chemical formulas will need every step! H2O Pb(NO3)2 2 KNO3 1. Identify all elements in the molecule/compound H O Pb N O K N O 2. Count the number of atoms of each element based on the subscript H= 2 O= 1 Pb= 1 N= 1 O= 3 K= 1 N= 1 O= 3 3. Get rid of parentheses by multiplying the number of atoms of each element INSIDE the parentheses by the subscript OUTSIDE the parentheses H= 2 O= 1 Pb= 1 K= 1 N= 1 x 2 = 2 N= 1 O= 3 x 2 = 6 O= 3 4. Multiply the number of atoms of ALL elements by the coefficient H= 2 O= 1 Pb= 1 N= 2 O= 6 K= 1 x 2 = 2 N= 1 x 2 = 2 O= 3 x 2 = 6 TOTALS H= 2 O= 1 Pb= 1 N= 2 O= 6 K= 2 N= 2 O= 6 3 Mg(OH)2 PRACTICE: Count the number of atoms of each element in the compound. 1. C10H14N2 (nicotine in cigarettes): C = _________ H = ___________ N = ________ 2. C6H8O7 (citric acid in orange, lemon): C= _______ H= ________ O = _________ 3. H3PO4 (phosphoric acid in soda) : H = ________ P= _________ O = _________ 4. 2 C2H4O2 (acetic acid/vinegar) : H = _______ C = _________ O = ___________ 5. H2CO3 (carbonic acid in soda) : H = ________ C = __________ O = _____________ 6. 5 H2SO4 (sulfuric acid in car batteries) : H= ________ S = ____________ O = ___________ 7. Al2(SO4)3 (water purification): Al= ________ S = ____________ O = _____________ 8. 3 Ca(OH)2 (calcium hydroxide- sewage treatment) : Ca= ________ O = _________ H = _________ 9. 4 CH4 (methane, flammable gas) : C = ___________ H = ___________ 10. (NH4)2SO4 (ammonium sulfate- fertilizer) : N= _______ H = _______ S =________ O= ________ 11. C18H27NO2 (capsaicin in chili pepper): C= ______ H = ______ N = ________ O = ________ 12. C2952 H4664 N812 O832 S8 Fe4 (hemoglobin in blood) : C = _____________ PRACTICE: Count the number of atoms of each element in the compound. 1. C10H14N2 (nicotine in cigarettes): C = _________ H = ___________ N = ________ 2. C6H8O7 (citric acid in orange, lemon): C= _______ H= ________ O = _________ 3. H3PO4 (phosphoric acid in soda) : H = ________ P= _________ O = _________ 4. 2 C2H4O2 (acetic acid/vinegar) : H = _______ C = _________ O = ___________ 5. H2CO3 (carbonic acid in soda) : H = ________ C = __________ O = _____________ 6. 5 H2SO4 (sulfuric acid in car batteries) : H= ________ S = ____________ O = ___________ 7. Al2(SO4)3 (water purification): Al= ________ S = ____________ O = _____________ 8. 3 Ca(OH)2 (calcium hydroxide- sewage treatment) : Ca= ________ O = _________ H = _________ 9. 4 CH4 (methane, flammable gas) : C = ___________ H = ___________ 10. (NH4)2SO4 (ammonium sulfate- fertilizer) : N= _______ H = _______ S =________ O= ________ 11. C18H27NO2 (capsaicin in chili pepper): C= ______ H = ______ N = ________ O = ________ 12. C2952 H4664 N812 O832 S8 Fe4 (hemoglobin in blood) : C = _______________