123.1 Required Training for Conducting Human Subject Research
advertisement
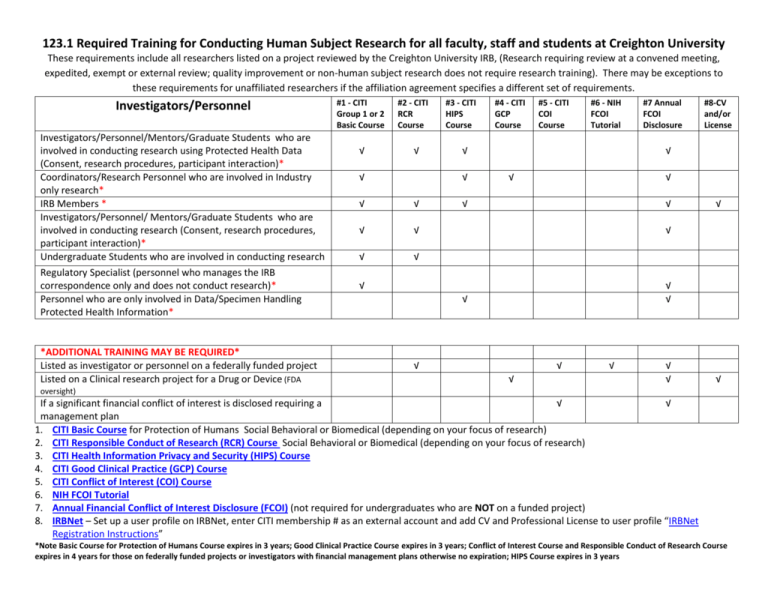
123.1 Required Training for Conducting Human Subject Research for all faculty, staff and students at Creighton University These requirements include all researchers listed on a project reviewed by the Creighton University IRB, (Research requiring review at a convened meeting, expedited, exempt or external review; quality improvement or non-human subject research does not require research training). There may be exceptions to these requirements for unaffiliated researchers if the affiliation agreement specifies a different set of requirements. Investigators/Personnel Investigators/Personnel/Mentors/Graduate Students who are involved in conducting research using Protected Health Data (Consent, research procedures, participant interaction)* Coordinators/Research Personnel who are involved in Industry only research* IRB Members * Investigators/Personnel/ Mentors/Graduate Students who are involved in conducting research (Consent, research procedures, participant interaction)* Undergraduate Students who are involved in conducting research Regulatory Specialist (personnel who manages the IRB correspondence only and does not conduct research)* Personnel who are only involved in Data/Specimen Handling Protected Health Information* *ADDITIONAL TRAINING MAY BE REQUIRED* Listed as investigator or personnel on a federally funded project Listed on a Clinical research project for a Drug or Device (FDA #1 - CITI Group 1 or 2 Basic Course √ #2 - CITI RCR Course √ √ #3 - CITI HIPS Course √ √ √ √ √ #5 - CITI COI Course #6 - NIH FCOI Tutorial √ √ √ #4 - CITI GCP Course #7 Annual FCOI Disclosure #8-CV and/or License √ √ √ √ √ √ √ √ √ √ √ √ √ √ √ √ √ √ oversight) If a significant financial conflict of interest is disclosed requiring a √ √ management plan 1. CITI Basic Course for Protection of Humans Social Behavioral or Biomedical (depending on your focus of research) 2. CITI Responsible Conduct of Research (RCR) Course Social Behavioral or Biomedical (depending on your focus of research) 3. CITI Health Information Privacy and Security (HIPS) Course 4. CITI Good Clinical Practice (GCP) Course 5. CITI Conflict of Interest (COI) Course 6. NIH FCOI Tutorial 7. Annual Financial Conflict of Interest Disclosure (FCOI) (not required for undergraduates who are NOT on a funded project) 8. IRBNet – Set up a user profile on IRBNet, enter CITI membership # as an external account and add CV and Professional License to user profile “IRBNet Registration Instructions” *Note Basic Course for Protection of Humans Course expires in 3 years; Good Clinical Practice Course expires in 3 years; Conflict of Interest Course and Responsible Conduct of Research Course expires in 4 years for those on federally funded projects or investigators with financial management plans otherwise no expiration; HIPS Course expires in 3 years