Florida State University College of Medicine CLINICAL RESEARCH
advertisement

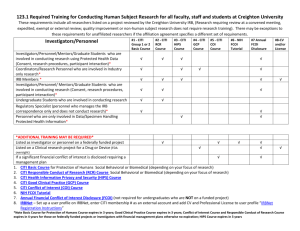
Florida State University College of Medicine CLINICAL RESEARCH NETWORK: Training on the Protection of Human Subjects in Research Welcome to the Florida State University College of Medicine Clinical Research Network! To foster and promote responsible and ethical research practices among our research community, the Florida State University College of Medicine requires certification in the responsible conduct of research. As a participant in the Clinical Research Network, you are provided free online training to meet this certification requirement, using the Collaborative Institutional Training Initiative (CITI) for the Protection of Human Subjects. The required CITI Courses include: Basic Course in the Protection of Human Research Subjects Health Information Privacy and Security Course (HIPS) About the CITI Course Self-guided, online courses Accepted by many medical schools, hospitals, health and medical institutions and funders Takes approximately three hours to complete; you can leave and re-enter the course at your convenience. You must obtain at least a 70% aggregate score in each of the two required courses to fulfill the training requirement. CITI website will generate a Completion Report for your records. Do not submit your Completion Report to the FSU College of Medicine. The FSU COM CITI Administrators will be able to view your Completion Report online. Certification is valid for three years, at which time a free refresher course will be available to you. Physicians can receive CMEs for a fee. Go to: http://www.citiprogram.org/citidocuments/cme/index.htm for information. Registration Instructions: To register, http://www.citiprogram.org/Default.asp. Click on “New Users Register Here”. Answer the seven registration questions: 1. SELECT YOUR INSTITUTION OR ORGANIZATION: To access the correct courses, select Florida State University College of Medicine as your institution, NOT Florida State University. 2. CREATE YOUR USERNAME AND PASSWORD. 3. SELECT A SECURITY QUESTION AND SUPPLY THE CORRECT ANSWER. 4. ENTER YOUR FIRST AND LAST NAME. 5. ENTER YOUR EMAIL ADDRESS. USE AN EMAIL ADDRESS THAT YOU WILL HAVE ACCESS TO IN THE FUTURE, AS NOTIFICATIONS FROM CITI REGARDING REFRESHER COURSE ARE SENT TO THE EMAIL ADDRESS YOU SUPPLY AT REGISTRATION. 6. CME/CEU CREDITS: if you want to receive CME or CEU credits from the CITI courses, you must select “yes” here and provide your professional affiliation. 7. COURSE SURVEY: if you want to complete a course survey, select “yes”. If you do not want to complete a course survey, select “no”. Click “Submit” at the bottom of the page. On the next page, you must answer at least the required fields*, which include: 1. INSTITUTIONAL EMAIL ADDRESS: Enter the same address entered on #5 above. 2. DEPARTMENT: Enter your academic department, hospital department or specialty. 3. ROLE IN RESEARCH: Select the first choice on the drop down list, “Clinical Researcher.” Select curriculum by answering the following three questions: Question 1: Human Subjects Research Select CRN/Clerkship Faculty Conducting Research with Pregnant Women, Fetuses or Neonates Question 2: Good Clinical Practice (GCP) Select Not at this time. (The Good Clinical Practice course is not required at this time.) Question 3: Health Information Privacy and Security (HIPS) Select HIPS Course for CRN/Clerkship Faculty Conducting Research in the Community Click on the “Submit” button at the bottom of the page. On the Main Menu, enter the courses by clicking “Not started - Enter” under My Courses Contact the CITI Administrators for the FSU College of Medicine with any questions or problems concerning the CITI Course on the Protection of Human Subjects. Jessica De Leon, PhD Phone: (850) 645-9702 Email: jessica.deleon@med.fsu.edu Michelle Vinson, MS RD LD/N Phone: (407) 835-4103 Email: michelle.vinson@med.fsu.edu