Research
advertisement
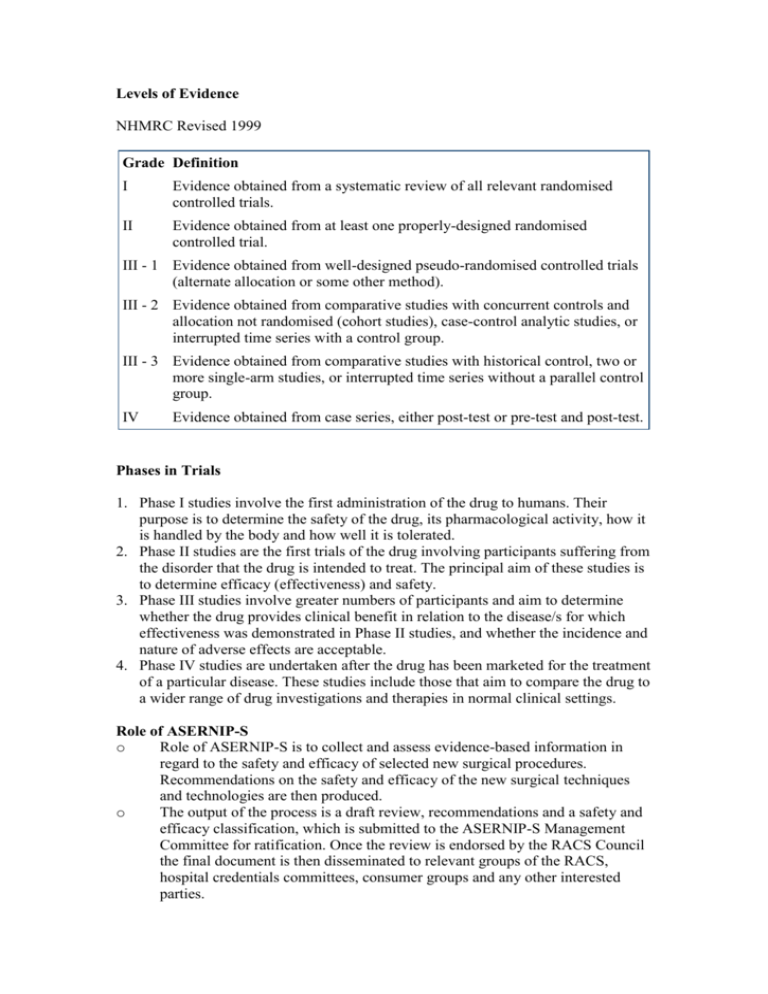
Levels of Evidence NHMRC Revised 1999 Grade Definition I Evidence obtained from a systematic review of all relevant randomised controlled trials. II Evidence obtained from at least one properly-designed randomised controlled trial. III - 1 Evidence obtained from well-designed pseudo-randomised controlled trials (alternate allocation or some other method). III - 2 Evidence obtained from comparative studies with concurrent controls and allocation not randomised (cohort studies), case-control analytic studies, or interrupted time series with a control group. III - 3 Evidence obtained from comparative studies with historical control, two or more single-arm studies, or interrupted time series without a parallel control group. IV Evidence obtained from case series, either post-test or pre-test and post-test. Phases in Trials 1. Phase I studies involve the first administration of the drug to humans. Their purpose is to determine the safety of the drug, its pharmacological activity, how it is handled by the body and how well it is tolerated. 2. Phase II studies are the first trials of the drug involving participants suffering from the disorder that the drug is intended to treat. The principal aim of these studies is to determine efficacy (effectiveness) and safety. 3. Phase III studies involve greater numbers of participants and aim to determine whether the drug provides clinical benefit in relation to the disease/s for which effectiveness was demonstrated in Phase II studies, and whether the incidence and nature of adverse effects are acceptable. 4. Phase IV studies are undertaken after the drug has been marketed for the treatment of a particular disease. These studies include those that aim to compare the drug to a wider range of drug investigations and therapies in normal clinical settings. Role of ASERNIP-S o Role of ASERNIP-S is to collect and assess evidence-based information in regard to the safety and efficacy of selected new surgical procedures. Recommendations on the safety and efficacy of the new surgical techniques and technologies are then produced. o The output of the process is a draft review, recommendations and a safety and efficacy classification, which is submitted to the ASERNIP-S Management Committee for ratification. Once the review is endorsed by the RACS Council the final document is then disseminated to relevant groups of the RACS, hospital credentials committees, consumer groups and any other interested parties. ASERNIP-S looks at these 3 areas Evidence rating 1. Poor 2. Average 3. Good Safety 1. At least as safe compared to comparator* procedure(s) 2. Safety cannot be determined 3. Less safe compared to comparator* procedure(s) Efficacy 1. At least as efficacious compared to comparator* procedure(s) 2. Efficacy cannot be determined 3. Less efficacious compared to comparator* procedure(s) *A comparator may be the current "gold standard" procedure, an alternative procedure, a non-surgical procedure or no treatment (natural history). If evidence poor, may recommend an audit be undertaken, or a controlled clinical trial. General Guidelines on Assessing, Approving and Introducing new technologies into a hospital Prior Evaluation o Has the technique been previously evaluated – literature and national bodies include: 1. INAHTA – International Network of Agencies for health technology assessment 2. ASERNIP-S – Australian Safety and Efficacy Register of New Interventional Procedures - Surgical, 3. Cochrane Collaboration 4. MSAC – Medical Services Advisory Committee o Training and experience within the institution and staff Approval Process o Principally hospital credentials committee, may also involve research and quality assurance committees. o RACS representative for advice if required o Clearance from hospital/regional ethics committee may be required o Need to set up outcome measures for evaluation o Individuals should declare any financial involvement that could result in a conflict of interest. o present and future costs of the new procedure should be estimated as accurately as possible o Resources/facilities of the region need to be assessed to see if the new procedure can be supported o Consider whether the hospital or health service will be able to do enough procedures per year to maintain necessary skill levels. Informed consent and Patient Information Conditional Approval may be given initially 1. Only by surgeons with a specified level of experience and expertise. 2. Only for certain indications. 3. Only for certain patient categories within a certain indication. 4. Only under certain experimental conditions e.g. in the context of a controlled clinical trial. Introduction and Monitoring o Learning curve should be avoided by using trained practitioners o Informed consent regarding new technology, alternatives o Monitoring – audit or trial o TGA should be advised if problems encountered



![Quality assurance in diagnostic radiology [Article in German] Hodler](http://s3.studylib.net/store/data/005827956_1-c129ff60612d01b6464fc1bb8f2734f1-300x300.png)






