Performance in Initiating Clinical Research – Q1 (2015
advertisement

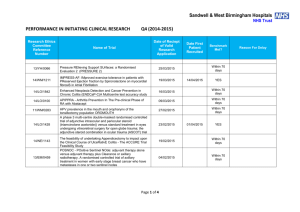
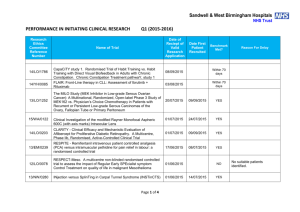
Sandwell & West Birmingham Hospitals NHS Trust PERFORMANCE IN INITIATING CLINICAL RESEARCH Research Ethics Committee Reference Number Q1 (2015-2016) Date of Reciept of Valid Research Application Name of Trial Date First Patient Recruited Benchmark Met? 13/EM/0239 RESPITE - Remifentanil intravenous patient controlled analgesia (PCA) versus intramuscular pethidine for pain relief in labour: a randomised controlled trial 17/06/2015 Within 70 days 12/LO/0078 RESPECT-Meso. A multicentre non-blinded randomised controlled trial to assess the impact of Regular Early SPEcialist symptom Control Treatment on quality of life in malignant Mesothelioma 01/06/2015 Within 70 days 13/NW/0280 INjection versus SplinTing in Carpal Tunnel Syndrome (INSTinCTS) 01/06/2015 Within 70 days 10/H0302/51 TB-REACH Virtually Observed Therapy (VOT) versus Directly Observed Therapy (DOT) 20/04/2015 14/EM/0121 RITUXILUP - An open label randomised multicentre controlled trial of RITUXImab and mycophenolate mofetil (MMF) without oral steroids for the treatment of LUPus nephritis 15/04/2015 14/LO/1043 LEAVO. A Multicentre Phase II Double-masked Randomised Controlled Non-Inferiority Trial Comparing the Clinical and Cost Effectiveness of Intravitreal Therapy with Ranibizumab (Lucentis) vs Aflibercept (Eylea) vs Bevacizumab (Avastin) for Macular Oedema due to Centre Retinal Vein Occlusion (CRVO). 13/04/2015 14/WM/0093 FOAM. A phase III randomised study of folic acid supplementation in the management of menopausal symptoms in cancer survivors and healthy postmenopausal women Page 1 of 4 01/04/2015 21/07/2015 03/06/2015 Reason For Delay NO No suitable patients identified. NO Staff shortages YES NO Restrictive eligibility criteria. Rrotocol amendment has been submitted to expand the potential participant population. 13/YH/0066 Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) 25/03/2015 14/WM/1211 IMPRESS-AF: IMproved exercise tolerance in patients with PReserved Ejection fraction by Spironolactone on myocardial fibrosiS in Atrial Fibrillation 19/03/2015 14/LO/1842 Enhanced Neoplasia Detection and Cancer Prevention in Chronic Colitis (ENDCaP-C)A Multicentre test accuracy study 16/03/2015 14/LO/0100 APIPPRA - Arthritis Prevention In The Pre-clinical Phase of RA with Abatacept 09/03/2015 11/WM/0283 HPV prevalence in the mouth and oropharynx of the tonsillectomy population OROMOUTH 27/02/2015 14/LO/1428 A phase 3 multi-centre double-masked randomised controlled trial of adjunctive intraocular and periocular steroid (triamcinolone acetonide)) versus standard treatment in eyes undergoing vitreoretinal surgery for open globe trauma; the adjunctive steroid combination in ocular trauma (ASCOT) trial 23/02/2015 14/NE/1143 The feasibility of undertaking Appendicectomy to impact upon the Clinical Course of UlceRativE Colitis - The ACCURE Trial Feasibility Study 19/02/2015 13/EM/0459 POSNOC - POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy. A randomised controlled trial of axillary treatment in women with early stage breast cancer who have metastases in one or two sentinel nodes 04/02/2015 14/NW/1351 Rehabilitation Enablement in Chronic Heart Failure (REACH HF). A multicentre parallel group randomised controlled trial with parallel economic and process evaluation to assess the clinical effectiveness and cost-effectiveness of the REACH HF manual for patients and caregivers. 23/01/2015 Page 2 of 4 NO 14/04/2015 No suitable patients identified. YES NO Grren light for study to open not given until 12 May 2015. First participant recruited within 70 days of green light NO Staff shortages 24/06/2015 NO Difficulties experienced in obtaining information about potential participants prior to tonsillectomy taking place 01/04/2015 YES 13/07/2015 NO Study closed at site due to PI leaving the Trust and no replacement PI being identified. 07/05/2015 NO Restrictive eligibility criteria which were amended in April 2015 and resulted in rapid recruitment of the first participant. 10/02/2015 YES 14/WM/0057 Multicentre randomised controlled trial to compare the clinical and costeffectiveness of a ‘vein bypass first’ with a ‘best endovascular first’ revascularisation strategy for severe limb ischaemia due to infrapopliteal arterial disease: Bypass vs. Angioplasty in Severe Ischaemia of the Leg. (BASIL- 2) 07/01/2015 13/EE/0339 A randomized controlled trial of rivaroxaban for the prevention of major cardiovascular events in patients with coronary or peripheral artery disease (COMPASS - Cardiovascular OutcoMes for People using Anticoagulation StrategieS) 07/01/2015 03/03/2015 YES 13/LO/0145 A multicentre phase III randomised controlled single masked clinical trial to test the clinical efficacy of LightMasks at preventing dark adaptation in the treatment of early diabetic macular oedema (CLEOPATRA) 17/12/2014 04/03/2015 NO Grren light for study to open not given until 10/02/015. First participant recruited within three weeks of green light NO Small patient population 13/LO0651 A randomised controlled trial of a biomarker-based exclusion of VAP to improve antibiotic stewardship. VAPrapid-2 08/12/2014 NO No suitable patients consented. Availability of staff at central laboratory has meant some suitable participants have not been consented. 13/SC/0111 FOCUS4. Molecular selection of therapy in colorectal cancer 05/12/2014 NO Site not activate until 04 June 2015 (6 months after submission). 14/WM/0159 Safety of Nasal Influenza Immunisation in Egg Allergic Children - The SNIFFLE 2 Study 03/11/2014 14/EM/1059 047 FIT - C-935788-047 A Phase 3, Multi-Center, Randomized, DoubleBlind, Placebo-Controlled, Study of Fostamatinib Disodium in the Treatment of Persistent/Chronic Immune Thrombocytopenic Purpura 30/10/2014 14/NW/0327 Effects of Glaucoma Surgery on Corneal Endothelial Celss - GICES 10/10/2014 14/NW/0130 RESPONSE: A randomised, double blind, multi-center, placebocontrolled study to evaluate the efficacy, safety, and tolerability of NT100 in pregnant women with a history of unexplained recurrent pregnancy loss (RPL) 07/10/2014 Page 3 of 4 17/12/2014 17/02/2015 YES NO Eligibility criteria prohibitive requiring very specific disease criteria. NO Staffing issues at site NO Study closed at site due to resource issues 12/SS/0138 REstart or STop Antithrombotics Randomised Trial (RESTART) 30/09/2014 28/11/2014 YES 13/EM/0395 Treatment of Advanced Glaucoma Study (TAGS) 01/10/2014 15/04/2015 NO 13/EM/0398 Phase II clinical trial investigating the use of epigallocatechin-3-gallate (Veregen) in the treatment of vulval intraepithelial neoplasia. EPIVIN trial (v 1.0) 17/09/2014 13/10/2014 YES 13/NW/0858 Assessment of an Education and Guidance programme for Eliquis Adherence in Non-Valvular Atrial Fibrillation (AEGEAN) 04/09/2014 22/10/2014 YES 14/EE/0102 ENSURE in AF (atrial fibrillation) study. A prospective, randomized, openlabel, blinded endpoint evaluation (PROBE) parallel group study comparing edoxaban (DU176b) with enoxaparin/warfarin followed by warfarin alone in subjects undergoing planned electrical cardioversion of nonvalvular atrial fibrillation 04/09/2014 20/10/2014 YES 12/WM/00335 A pilot study for developing and evaluating a care pathway for cognitive problems after stroke (OCS-care) 28/07/2014 28/08/2014 YES 14/WS/0004 Open-Label Extension Study of EFC12492, R727-CL-1112, EFC12732, & LTS11717 Studies to Assess the Long-Term Safety and Efficacy of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia ODYSSEY OLE 22/07/2014 25/09/2014 YES 14/WM/0083 A Phase III Trial of Surgery versus Active Monitoring for Low Risk Ductal Carcinoma in Situ. The Low Risk DCIS Trial (LORIS). 23/07/2014 14/08/2014 YES 14/EE/0059 Post-Market Multicentric Evaluation of the AQUESYS XEN Implant in Moderate Primary Open Angle Glaucoma Subjects. MS-001 Study 18/07/2014 10/09/2014 YES 14/EE/0102 SPIRE 2: B1481038 Phase 3 multi-center, double-blind, randomized, Placebo-controlled, parallel group evaluation of the Efficacy, safety, and tolerability of pf-04950615, in reducing the Occurrence of major cardiovascular events in high risk Subjects 10/07/2014 13/EM/0427 MiQuit trial: Tailored text messages for pregnant women v1 03/07/2014 Page 4 of 4 NO 29/07/2014 YES No suitable patients identified. Sponsor delays: Green light to start the study not given until 18/12/2014. Eligibility criteria prohibitive requiring very specific disease criteria.