Terminology and Concepts Liquid and Solid States (Part 4). - E
advertisement

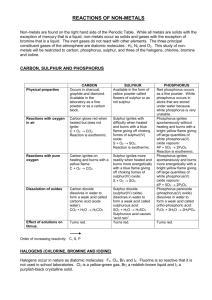
STPM Chemistry Form 6 Notes – Terminology and Concepts: Liquid and Solid States (Part 4) The concept of allotropy was proposed in 1841 by the Swedish scientist Baron Jöns Jakob Berzelius (1779-1848). The term is derived from the Greek άλλοτροπἱα (allotropia; variation, changeableness). By 1912, Ostwald proposed that the terms allotrope and allotropy be abandoned and replaced by polymorph and polymorphism but IUPAC and most chemistry texts still favour the usage of allotrope and allotropy for elements only. Allotropy – existence of elements in two or more different forms (allotropes). Elements with variable of coordination number or oxidation states tend to exhibit greater numbers of allotropic forms and typically more noticeable in non-metal (excluding the halogens and the noble gases) and metalloids. Example: i) Different molecular configuration Oxygen – O2 dioxygen (colourless), O3 trioxygen / ozone (blue), O4 tetraoxygen, O8octaoxygen (red) ii) Different crystal structures in the solid Group 14, Group 15, Group 16 of the periodic table Group 14: Carbon – graphite, amorphous carbon (soot/coal), diamond, fullerenes C60(buckyball), Ionsdaleite / hexagonal diamond (meteorites containing graphite strike to the Earth) and carbon nanotubes (buckytubes) is carbon with a cylindrical nanostructure. Group 15: Phosphorus – red phosphorus (polymeric solid), white phosphorus (crystalline solid P4), scarlet phosphorus, violet phosphorus, black phosphorus (semiconductor) and diphosphorus P2. Group 16: Sulphur – rhombic sulphur (large crystals composed of S8 molecules), monoclinic sulphur (fine needle-like crystals), plastic (amorphous) sulphur (polymeric solid) and other ring molecules S7 and S12. Enantiotropy – the allotropes are stable over a temperature range, with a definite transitionpoint at which one changes into the other. Example: i) Tin has three allotropes / two enantiotropy: alpha tin is white (metallic) tin stable above 13.2 ˚C. beta tin is grey (nonmethallic) tin below 13.2 ˚C. gamma tin is rhombic tin. ii) Iron has four allotropes / four enantiotropy: ferrite (alpha iron) stable below 770°C (BCC) and the iron becomes magnetic. beta iron stable below 912°C (BCC). gamma irons stable below 1394°C (FCC) crystal structure. delta irons stable from cooling down molten iron below 1538°C and has a (BCC) crystal structure. *BCC – Body-centred cubic *FCC – Face-centred cubic Allotropes of Carbon i) Graphite – used as lubricant (powder or oily suspension) layered lattice structure hexoganal for the crystal system density is 2.25 g cm-3 each carbon atom is bonded by strong covalent bonds (sp2 hybridisation / trigonal planar) with three other carbon atoms to formed hexagonal ring. the layer are held together by weak van der Waals forces. graphite is soft and slippery due to weak van der Waals forces allow the layer to slide over one another. graphite is a moderate conductor of electricity along its layer (in the direction parallel but not perpendicular to the laver) due to a free electron (per carbon atom) which can move throughout the solid lattice. (Each carbon atom has one outer shell electron (unhybridised p electron) which is not used to form covalent bonds.) ii) Diamond – used as abrasives (high velocity cutting tools) and ornaments (high refractive index) crystallises in a face-centred cubic structure. single giant molecule. density is 3.50 g cm-3 each carbon atom is bonded by strong covalent bonds (sp3 hybridisation / tetrahedral) withfour other carbon atoms to formed three-dimensional giant structure. diamond has great hardness and high melting point due to the strong covalent bonds in the 3-D structure. diamond is a non-conductor of electricity due to all the four valence electrons of the carbon atoms are involved with covalent bonding, therefore no free/delocalised electrons. iii) Fullerene / Buckyball / Buckminsterfullerene – used as lubricant, semiconductor, superconductors and catalyst Molecular formulae of fullerene are C20 (smallest member), C32, C60 (most common member), C70, C76, C78, C84 and C90. spherical molecules of 20 – 90 carbon atoms (32 sides, 12 pentagons and 20 hexagons). simple molecular solid. each carbon atom is bonded by strong covalent bonds (sp2 hybridisation / trigonal planar) with three other carbon atoms. It also contains delocalised π electrons which does not exhibit “superaromaticity” that the electrons in the hexagonal rings do not delocalise over the whole molecule. Fullerene is a superconductor when it mixed with other metals. Allotropes of Sulphur (different molecular arrangement) i) alpha sulphur / rhombic sulphur (large crystals composed of S8 molecules) lemon yellow colour shape of an octahedron. crystallises with the orthorhombic lattice. more stable at room temperature (formed in temperature below 95.6˚C). melting point at 113˚C. density is 2.07 g cm3. ii) beta sulphur / monoclinic sulphur (fine needle-like crystals of S8 molecules) deeper yellow colour shape of long, narrow and thin needle. crystallises with the monoclinic lattice. stable at temperature above 95.6˚C. melting point at 119˚C. density is 1.94 g cm3.