reactions of non-metals
advertisement
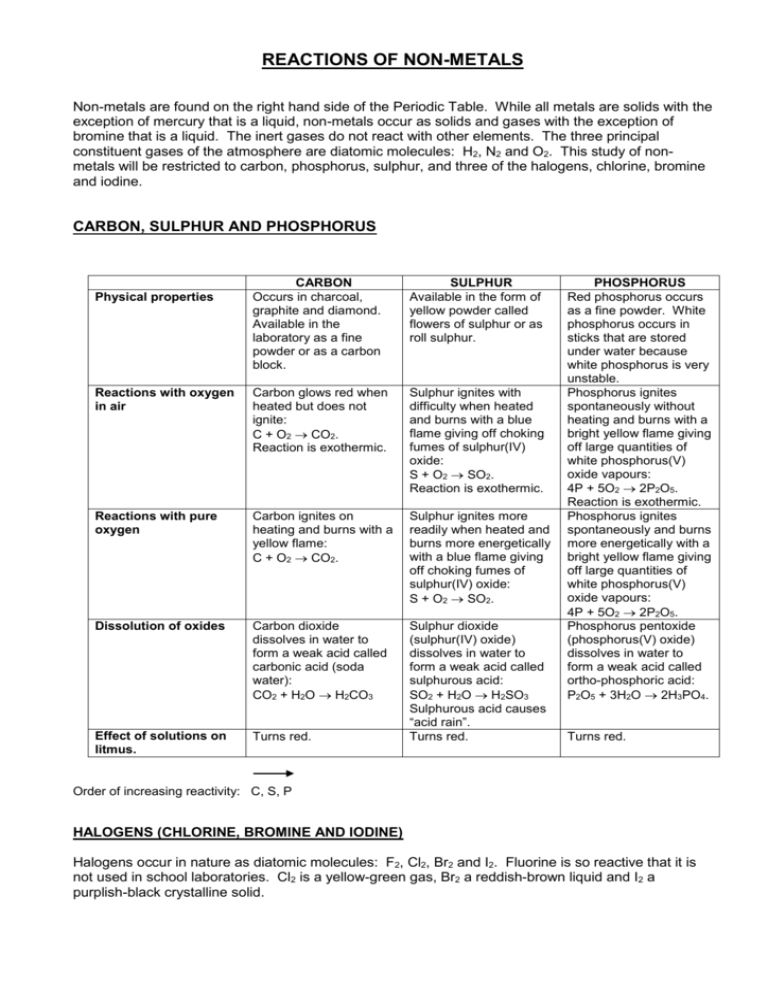
REACTIONS OF NON-METALS Non-metals are found on the right hand side of the Periodic Table. While all metals are solids with the exception of mercury that is a liquid, non-metals occur as solids and gases with the exception of bromine that is a liquid. The inert gases do not react with other elements. The three principal constituent gases of the atmosphere are diatomic molecules: H2, N2 and O2. This study of nonmetals will be restricted to carbon, phosphorus, sulphur, and three of the halogens, chlorine, bromine and iodine. CARBON, SULPHUR AND PHOSPHORUS CARBON Occurs in charcoal, graphite and diamond. Available in the laboratory as a fine powder or as a carbon block. SULPHUR Available in the form of yellow powder called flowers of sulphur or as roll sulphur. Reactions with oxygen in air Carbon glows red when heated but does not ignite: C + O2 CO2. Reaction is exothermic. Sulphur ignites with difficulty when heated and burns with a blue flame giving off choking fumes of sulphur(IV) oxide: S + O2 SO2. Reaction is exothermic. Reactions with pure oxygen Carbon ignites on heating and burns with a yellow flame: C + O2 CO2. Sulphur ignites more readily when heated and burns more energetically with a blue flame giving off choking fumes of sulphur(IV) oxide: S + O2 SO2. Dissolution of oxides Carbon dioxide dissolves in water to form a weak acid called carbonic acid (soda water): CO2 + H2O H2CO3 Effect of solutions on litmus. Turns red. Sulphur dioxide (sulphur(IV) oxide) dissolves in water to form a weak acid called sulphurous acid: SO2 + H2O H2SO3 Sulphurous acid causes “acid rain”. Turns red. Physical properties PHOSPHORUS Red phosphorus occurs as a fine powder. White phosphorus occurs in sticks that are stored under water because white phosphorus is very unstable. Phosphorus ignites spontaneously without heating and burns with a bright yellow flame giving off large quantities of white phosphorus(V) oxide vapours: 4P + 5O2 2P2O5. Reaction is exothermic. Phosphorus ignites spontaneously and burns more energetically with a bright yellow flame giving off large quantities of white phosphorus(V) oxide vapours: 4P + 5O2 2P2O5. Phosphorus pentoxide (phosphorus(V) oxide) dissolves in water to form a weak acid called ortho-phosphoric acid: P2O5 + 3H2O 2H3PO4. Turns red. Order of increasing reactivity: C, S, P HALOGENS (CHLORINE, BROMINE AND IODINE) Halogens occur in nature as diatomic molecules: F2, Cl2, Br2 and I2. Fluorine is so reactive that it is not used in school laboratories. Cl2 is a yellow-green gas, Br2 a reddish-brown liquid and I2 a purplish-black crystalline solid. TEST FOR HALOGENS Halogens can be identified by the colour of a solution of the halogen in carbon disulphide, carbon tetrachloride or chloroform: Chlorine - colourless Bromine - yellow-brown Iodine – purple. CS2 sinks to the bottom of the test tube because it is denser than water. (Molar mass of CS 2 = 76 g.mol-1 compared with the molar mass of H2O = 18 g.mol-1.) REACTION WITH METALS The reactions between iodine and the metals sodium and zinc will be studied. Sodium reacts spontaneously with iodine burning with a yellow flame and giving off purple iodine vapours while yellow sparks shoot out in all directions: 2Na + I2 2NaI. The reaction is exothermic. Zinc reacts with iodine after a drop of water has been added as a catalyst: Zn + I 2 ZnI2. The reaction is exothermic. Sodium is more reactive than zinc. The products formed are called halides. For example, the halogen, iodine, becomes the halide, iodide. DISPLACEMENT OF HALIDES BY HALOGENS Since chlorine and bromine are very toxic, chlorine water and bromine water are used for reactions in the laboratory. A halogen can displace any other halogen that appears lower down in the reactivity series from a halide solution. Reactivity series for halogens: F2 Cl2 Br2 I2 KCl (aq) KBr (aq) KI (aq) Cl2 x Br2 x x Cl2 + 2KBr 2KCl + Br2 Cl2 + 2KI 2KCl + I2 Br2 + 2KI 2KBr + I2