Compounds
advertisement
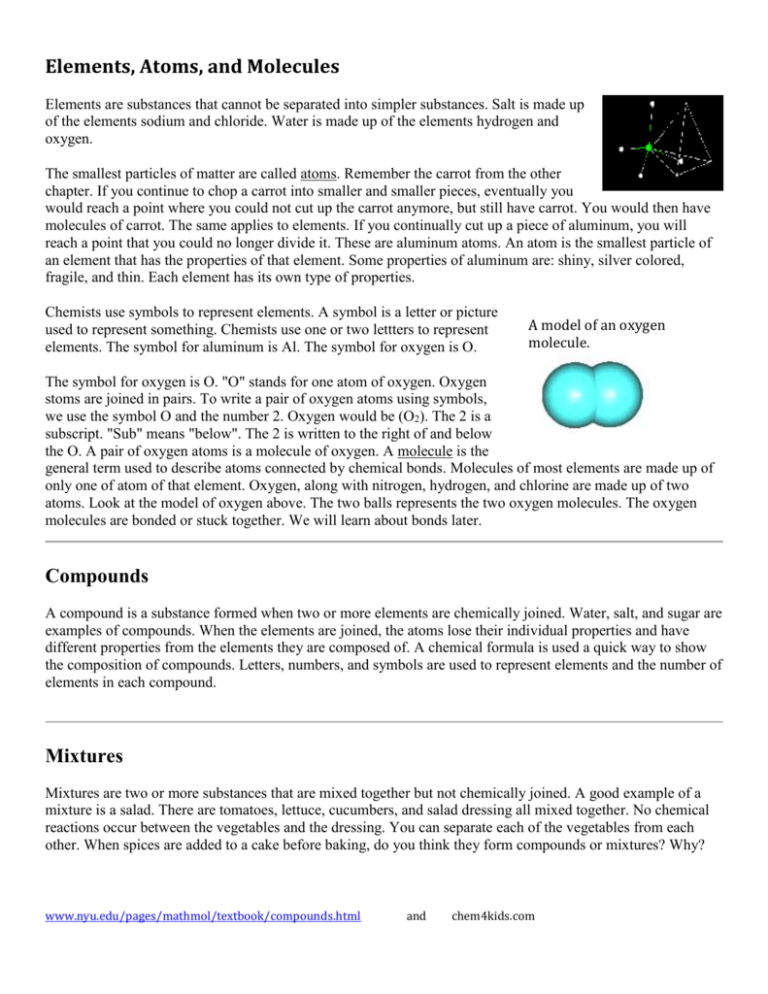
Elements, Atoms, and Molecules Elements are substances that cannot be separated into simpler substances. Salt is made up of the elements sodium and chloride. Water is made up of the elements hydrogen and oxygen. The smallest particles of matter are called atoms. Remember the carrot from the other chapter. If you continue to chop a carrot into smaller and smaller pieces, eventually you would reach a point where you could not cut up the carrot anymore, but still have carrot. You would then have molecules of carrot. The same applies to elements. If you continually cut up a piece of aluminum, you will reach a point that you could no longer divide it. These are aluminum atoms. An atom is the smallest particle of an element that has the properties of that element. Some properties of aluminum are: shiny, silver colored, fragile, and thin. Each element has its own type of properties. Chemists use symbols to represent elements. A symbol is a letter or picture used to represent something. Chemists use one or two lettters to represent elements. The symbol for aluminum is Al. The symbol for oxygen is O. A model of an oxygen molecule. The symbol for oxygen is O. "O" stands for one atom of oxygen. Oxygen stoms are joined in pairs. To write a pair of oxygen atoms using symbols, we use the symbol O and the number 2. Oxygen would be (O2). The 2 is a subscript. "Sub" means "below". The 2 is written to the right of and below the O. A pair of oxygen atoms is a molecule of oxygen. A molecule is the general term used to describe atoms connected by chemical bonds. Molecules of most elements are made up of only one of atom of that element. Oxygen, along with nitrogen, hydrogen, and chlorine are made up of two atoms. Look at the model of oxygen above. The two balls represents the two oxygen molecules. The oxygen molecules are bonded or stuck together. We will learn about bonds later. Compounds A compound is a substance formed when two or more elements are chemically joined. Water, salt, and sugar are examples of compounds. When the elements are joined, the atoms lose their individual properties and have different properties from the elements they are composed of. A chemical formula is used a quick way to show the composition of compounds. Letters, numbers, and symbols are used to represent elements and the number of elements in each compound. Mixtures Mixtures are two or more substances that are mixed together but not chemically joined. A good example of a mixture is a salad. There are tomatoes, lettuce, cucumbers, and salad dressing all mixed together. No chemical reactions occur between the vegetables and the dressing. You can separate each of the vegetables from each other. When spices are added to a cake before baking, do you think they form compounds or mixtures? Why? www.nyu.edu/pages/mathmol/textbook/compounds.html and chem4kids.com Name: ______________________________________________ Date: ____________ E# ________ The Makeup of Matter: www.youtube.com/watch?v=cV4jJZCIMPo Follow up: www.youtube.com/watch?v=AfXxZwNLvPA Atom: “That which cannot be cut/indivisible” 1) What is an atom? 2) What is meant by a “pure substance”? 3) How does an atom compare to an element? 4) Are atoms made up of elements? 5) Are elements made up of atoms YES YES NO NO 6) How many different elements are there? 7) What makes something a molecule? 8) What makes something a compound? 9) How is a compound different than a mixture? 10) Are compounds made up of molecules? YES NO 11) Are molecules made up of compounds? YES NO SUMMARY When matter is made of only ONE kind of atom, it is called a(n) ________________________. When matter is a combination of two or more kinds of atoms, it is called a(n) _______________ When matter is a combination of two or more kinds of elements, it is called a(n) _________________ Name: ______________________________________________ Date: ____________ E# ________ The Makeup of Matter: www.youtube.com/watch?v=cV4jJZCIMPo Follow up: www.youtube.com/watch?v=AfXxZwNLvPA Atom: “That which cannot be cut/indivisible” 1) What is an atom? Smallest particle of all matter 2) What is meant by a “pure substance” Substance only contains ONE kind of particle 3) How does an atom compare to an element? All elements are made up of the SAME kind of atom 4) Are atoms made up of elements? YES NO 5) Are elements made up of atoms YES NO 6) How many different elements are there? 116-117 7) What makes something a molecule? Two or more atoms CHEMICALLY combined 8) What makes something a compound? A substance made of two or more elements. 9) How is a compound different than a mixture? A compound is formed when elements chemically combine. Compound have new properties from original substances. Mixture contains unique elements or molecules that still have their original properties. The elements and molecules can be separated (like salt from water) 10) Are compounds made up of molecules? YES NO 11) Are molecules made up of compounds? YES NO SUMMARY When matter is made of only ONE kind of atom, it is called a(n) _________element. When matter is a combination of two or more kinds of atoms, it is called a(n) ________ molecule When matter is a combination of two or more kinds of elements, it is called a(n) _____Compound